Chapter 9 - University of Dayton Academic Webserver
Chapter 9 - University of Dayton Academic Webserver
Chapter 9 - University of Dayton Academic Webserver
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chapter</strong> 9: Molecular Structures 325<br />
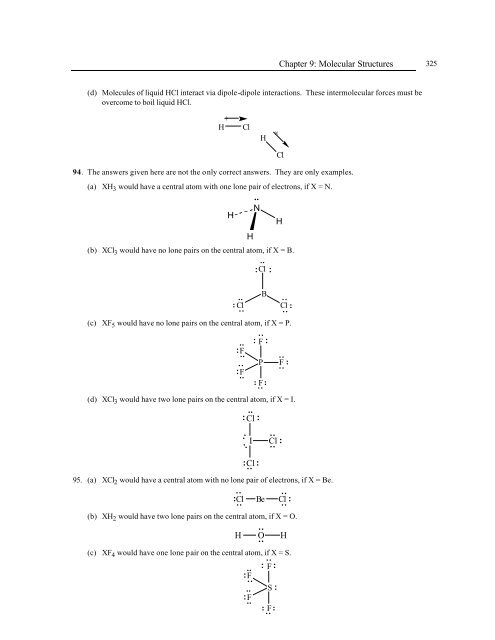
(d) Molecules <strong>of</strong> liquid HCl interact via dipole-dipole interactions. These intermolecular forces must be<br />
overcome to boil liquid HCl.<br />
+<br />
H Cl<br />
94. The answers given here are not the only correct answers. They are only examples.<br />
(a) XH 3 would have a central atom with one lone pair <strong>of</strong> electrons, if X = N.<br />
(b) XCl 3 would have no lone pairs on the central atom, if X = B.<br />
H<br />
. ..<br />
..<br />
H<br />
.<br />
N<br />
H<br />
+<br />
..<br />
. Cl .<br />
Cl<br />
H<br />
..<br />
.. .<br />
B<br />
Cl Cl<br />
(c) XF 5 would have no lone pairs on the central atom, if X = P.<br />
..<br />
. F..<br />
.<br />
.<br />
. F.<br />
. . .<br />
.<br />
F<br />
P<br />
. . F.<br />
.<br />
. .<br />
F .<br />
. .<br />
(d) XCl 3 would have two lone pairs on the central atom, if X = I.<br />
. .<br />
.<br />
Cl<br />
..<br />
.<br />
I<br />
. Cl . .<br />
.<br />
. .<br />
Cl .<br />
. .<br />
95. (a) XCl 2 would have a central atom with no lone pair <strong>of</strong> electrons, if X = Be.<br />
..<br />
. Cl ..<br />
..<br />
.. .<br />
Be Cl<br />
(b) XH 2 would have two lone pairs on the central atom, if X = O.<br />
H<br />
. .<br />
. .<br />
O H<br />
(c) XF 4 would have one lone pair on the central atom, if X = S.<br />
..<br />
. F..<br />
. . . F.<br />
. . .<br />
.<br />
F<br />
S<br />
.<br />
. . F.<br />
.