chapter 2 - Bentham Science
chapter 2 - Bentham Science
chapter 2 - Bentham Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Cyclopeptide-Based Glycoclusters Synthesis and Biological Applications of Glycoconjugates 131<br />
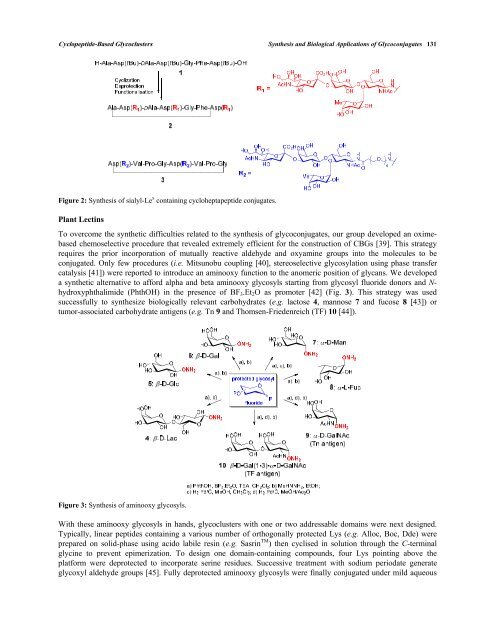
Figure 2: Synthesis of sialyl-Le x containing cycloheptapeptide conjugates.<br />
Plant Lectins<br />
To overcome the synthetic difficulties related to the synthesis of glycoconjugates, our group developed an oximebased<br />
chemoselective procedure that revealed extremely efficient for the construction of CBGs [39]. This strategy<br />
requires the prior incorporation of mutually reactive aldehyde and oxyamine groups into the molecules to be<br />
conjugated. Only few procedures (i.e. Mitsunobu coupling [40], stereoselective glycosylation using phase transfer<br />
catalysis [41]) were reported to introduce an aminooxy function to the anomeric position of glycans. We developed<br />
a synthetic alternative to afford alpha and beta aminooxy glycosyls starting from glycosyl fluoride donors and Nhydroxyphthalimide<br />
(PhthOH) in the presence of BF3.Et2O as promoter [42] (Fig. 3). This strategy was used<br />
successfully to synthesize biologically relevant carbohydrates (e.g. lactose 4, mannose 7 and fucose 8 [43]) or<br />
tumor-associated carbohydrate antigens (e.g. Tn 9 and Thomsen-Friedenreich (TF) 10 [44]).<br />
Figure 3: Synthesis of aminooxy glycosyls.<br />
With these aminooxy glycosyls in hands, glycoclusters with one or two addressable domains were next designed.<br />
Typically, linear peptides containing a various number of orthogonally protected Lys (e.g. Alloc, Boc, Dde) were<br />
prepared on solid-phase using acido labile resin (e.g. Sasrin TM ) then cyclised in solution through the C-terminal<br />
glycine to prevent epimerization. To design one domain-containing compounds, four Lys pointing above the<br />
platform were deprotected to incorporate serine residues. Successive treatment with sodium periodate generate<br />
glycoxyl aldehyde groups [45]. Fully deprotected aminooxy glycosyls were finally conjugated under mild aqueous