chapter 2 - Bentham Science
chapter 2 - Bentham Science
chapter 2 - Bentham Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
242 Synthesis and Biological Applications of Glycoconjugates Nativi et al.<br />
’-Dioxothiones as Electron-Poor Dienes<br />
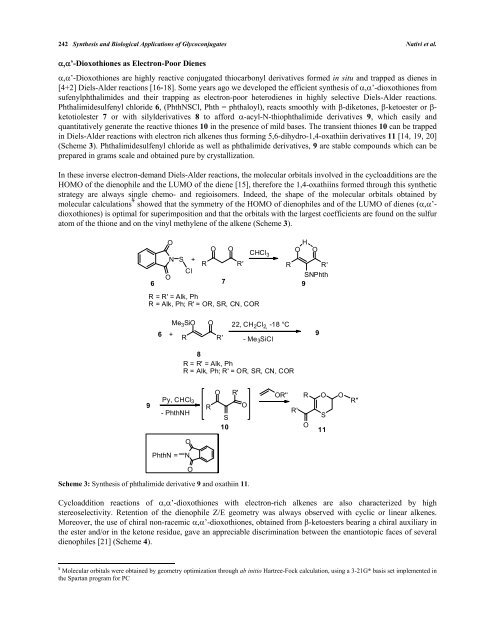
’-Dioxothiones are highly reactive conjugated thiocarbonyl derivatives formed in situ and trapped as dienes in<br />
[4+2] Diels-Alder reactions [16-18]. Some years ago we developed the efficient synthesis of ’-dioxothiones from<br />
sufenylphthalimides and their trapping as electron-poor heterodienes in highly selective Diels-Alder reactions.<br />
Phthalimidesulfenyl chloride 6, (PhthNSCl, Phth = phthaloyl), reacts smoothly with -diketones, -ketoester or ketotiolester<br />
7 or with silylderivatives 8 to afford -acyl-N-thiophthalimide derivatives 9, which easily and<br />
quantitatively generate the reactive thiones 10 in the presence of mild bases. The transient thiones 10 can be trapped<br />
in Diels-Alder reactions with electron rich alkenes thus forming 5,6-dihydro-1,4-oxathiin derivatives 11 [14, 19, 20]<br />
(Scheme 3). Phthalimidesulfenyl chloride as well as phthalimide derivatives, 9 are stable compounds which can be<br />
prepared in grams scale and obtained pure by crystallization.<br />
In these inverse electron-demand Diels-Alder reactions, the molecular orbitals involved in the cycloadditions are the<br />
HOMO of the dienophile and the LUMO of the diene [15], therefore the 1,4-oxathiins formed through this synthetic<br />
strategy are always single chemo- and regioisomers. Indeed, the shape of the molecular orbitals obtained by<br />
molecular calculations ¥ showed that the symmetry of the HOMO of dienophiles and of the LUMO of dienes (’dioxothiones)<br />
is optimal for superimposition and that the orbitals with the largest coefficients are found on the sulfur<br />
atom of the thione and on the vinyl methylene of the alkene (Scheme 3).<br />
6<br />
Scheme 3: Synthesis of phthalimide derivative 9 and oxathiin 11.<br />
O<br />
O<br />
N<br />
O O<br />
S +<br />
Cl<br />
R R'<br />
R=R'=Alk,Ph<br />
R = Alk, Ph; R' = OR, SR, CN, COR<br />
9<br />
6 +<br />
PhthN =<br />
Me 3SiO O<br />
R<br />
Py, CHCl3<br />
- PhthNH<br />
O<br />
N<br />
O<br />
R<br />
R'<br />
O<br />
7<br />
S<br />
10<br />
O<br />
CHCl 3<br />
22, CH 2Cl 2, -18 °C<br />
-Me3SiCl<br />
8<br />
R=R'=Alk,Ph<br />
R=Alk,Ph;R'=OR,SR,CN,COR<br />
R' OR''<br />
H<br />
O O<br />
R R'<br />
SNPhth<br />
9<br />
Cycloaddition reactions of ’-dioxothiones with electron-rich alkenes are also characterized by high<br />
stereoselectivity. Retention of the dienophile Z/E geometry was always observed with cyclic or linear alkenes.<br />
Moreover, the use of chiral non-racemic ’-dioxothiones, obtained from -ketoesters bearing a chiral auxiliary in<br />
the ester and/or in the ketone residue, gave an appreciable discrimination between the enantiotopic faces of several<br />
dienophiles [21] (Scheme 4).<br />
¥<br />
Molecular orbitals were obtained by geometry optimization through ab initio Hartree-Fock calculation, using a 3-21G* basis set implemented in<br />
the Spartan program for PC<br />
R'<br />
R<br />
O<br />
9<br />
O<br />
S<br />
11<br />
O R''