Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
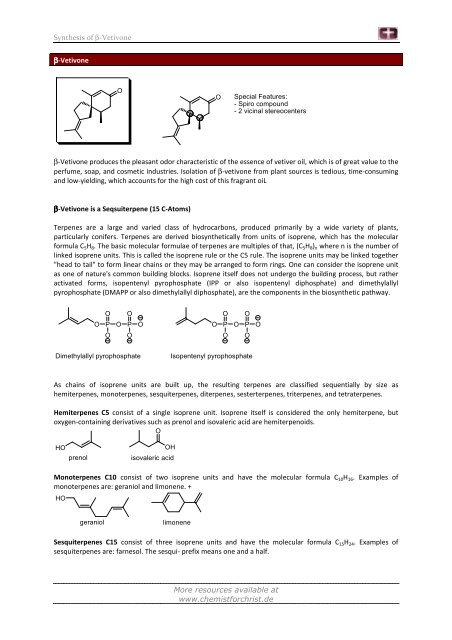
<strong>β</strong>-<strong>Vetivone</strong><br />
O<br />
O Special Features:<br />
- Spiro compound<br />
- 2 vicinal stereocenters<br />
<strong>β</strong>-<strong>Vetivone</strong> produces the pleasant odor characteristic <strong>of</strong> the essence <strong>of</strong> vetiver oil, which is <strong>of</strong> great value to the<br />
perfume, soap, and cosmetic industries. Isolation <strong>of</strong> <strong>β</strong>-vetivone from plant sources is tedious, time-consuming<br />
and low-yielding, which accounts for the high cost <strong>of</strong> this fragrant oiL<br />
<strong>β</strong>-<strong>Vetivone</strong> is a Seqsuiterpene (15 C-Atoms)<br />
Terpenes are a large and varied class <strong>of</strong> hydrocarbons, produced primarily by a wide variety <strong>of</strong> plants,<br />
particularly conifers. Terpenes are derived biosynthetically from units <strong>of</strong> isoprene, which has the molecular<br />
formula C5H8. The basic molecular formulae <strong>of</strong> terpenes are multiples <strong>of</strong> that, (C5H8)n where n is the number <strong>of</strong><br />
linked isoprene units. This is called the isoprene rule or the C5 rule. The isoprene units may be linked together<br />
"head to tail" to form linear chains or they may be arranged to form rings. One can consider the isoprene unit<br />
as one <strong>of</strong> nature's common building blocks. Isoprene itself does not undergo the building process, but rather<br />
activated forms, isopentenyl pyrophosphate (IPP or also isopentenyl diphosphate) and dimethylallyl<br />
pyrophosphate (DMAPP or also dimethylallyl diphosphate), are the components in the biosynthetic pathway.<br />
O<br />
O P O<br />
O<br />
O<br />
P O<br />
O<br />
O P O<br />
Dimethylallyl pyrophosphate Isopentenyl pyrophosphate<br />
O<br />
O<br />
As chains <strong>of</strong> isoprene units are built up, the resulting terpenes are classified sequentially by size as<br />
hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, and tetraterpenes.<br />
Hemiterpenes C5 consist <strong>of</strong> a single isoprene unit. Isoprene itself is considered the only hemiterpene, but<br />
oxygen-containing derivatives such as prenol and isovaleric acid are hemiterpenoids.<br />
O<br />
HO<br />
prenol<br />
OH<br />
isovaleric acid<br />
Monoterpenes C10 consist <strong>of</strong> two isoprene units and have the molecular formula C10H16. Examples <strong>of</strong><br />
monoterpenes are: geraniol and limonene. +<br />
HO<br />
geraniol limonene<br />
Sesquiterpenes C15 consist <strong>of</strong> three isoprene units and have the molecular formula C15H24. Examples <strong>of</strong><br />
sesquiterpenes are: farnesol. The sesqui- prefix means one and a half.<br />
P O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
O
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
HO<br />
farnesol<br />
Diterpenes C20 are composed for four isoprene units and have the molecular formula C20H32. They derive from<br />
geranylgeranyl pyrophosphate. Examples <strong>of</strong> diterpenes are cafestol, kahweol, cembrene and taxadiene<br />
(precursor <strong>of</strong> taxol). Diterpenes also form the basis for biologically important compounds such as retinol,<br />
retinal, and phytol. They are known to be antimicrobial and antiinflammatory.<br />
Triterpenes C30 consist <strong>of</strong> six isoprene units and have the molecular formula C30H48. The linear triterpene<br />
squalene, the major constituent <strong>of</strong> shark liver oil, is derived from the reductive coupling <strong>of</strong> two molecules <strong>of</strong><br />
farnesyl pyrophosphate. Squalene is then processed biosynthetically to generate either lanosterol or<br />
cycloartenol, the structural precursors to all the steroids.<br />
squalene<br />
Polyterpenes consist <strong>of</strong> long chains <strong>of</strong> many isoprene units. Natural rubber consists <strong>of</strong> polyisoprene in which<br />
the double bonds are cis. Some plants produce a polyisoprene with trans double bonds, known as guttapercha.<br />
Correct structure elucidation by M.J. Marshall JACS 1967, 89, 2749 and 2750<br />
First synthesis: M.J. Marshall JOC 1970, 35, 192<br />
Possible synthesis <strong>of</strong> the starting material used by Marshall:<br />
O O<br />
Key-step reaction:<br />
Base<br />
stays axial to avoid A 1.2 strain<br />
with OH<br />
Excerpt from a textbook how the mechanism could go:<br />
The Di-π-Methane rearrangement<br />
3<br />
hν<br />
O<br />
O<br />
Me<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
hν, dioxane<br />
1,4 Dienes carrying alkyl or aryl Substituents on C-3 can be photochemically rearraanget to vinylcyclopropane<br />
in a reaction that is called the Di-π-Methane rearrangement. When photolyzed 2,5-cyclohexadienones can<br />
undergo a number <strong>of</strong> different reactions, one <strong>of</strong> which is formally the same as the di-π-methane<br />
rearrangement.<br />
O<br />
O
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
O<br />
Ph Ph<br />
hν<br />
O<br />
Ph Ph<br />
O<br />
Ph Ph<br />
Another proposal for the mechanism (ionic pathway):<br />
KROPP, JACS, 1963, 85, 3779<br />
O<br />
O<br />
hν, dioxane<br />
hν, dioxane<br />
O<br />
O<br />
Ph Ph<br />
O O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
Ph Ph<br />
AcOH, Ac 2O<br />
H 2SO 4<br />
In comparison with the desired molecule, following steps have to be done:<br />
Get rid <strong>of</strong> C=O<br />
O<br />
Attach:<br />
Introduce a C=O<br />
Remember: This is chemistry from 1970, fancy reagents haven't been known then and benzene was still used<br />
as a solvent!<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Ph<br />
H<br />
Ph
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
O<br />
n-BuSH<br />
Et 2O<br />
H 2SO 4<br />
O<br />
BuS<br />
AcO<br />
AcO<br />
O<br />
H OMe<br />
Base<br />
O<br />
HO<br />
NaBH 4,<br />
MeOH<br />
Ac 2O,<br />
NaOAc, Δ<br />
Na 2CrO 4, AcOH<br />
Ac 2O, 40°C<br />
O<br />
O<br />
O<br />
HO<br />
BuS<br />
HO<br />
BF 3 . Et2O, rt<br />
O<br />
O<br />
O<br />
<strong>β</strong>−<strong>Vetivone</strong><br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
HgCl 2, H 3O +<br />
acetone<br />
MeLi, Et 2O<br />
- 35°C<br />
O<br />
O<br />
O<br />
O<br />
hν, dioxane<br />
, NaH<br />
H OEt<br />
benzene, MeOH<br />
O<br />
O<br />
MeLi, Et 2O<br />
- 35°C<br />
1. Li, NH 3, Et 2O<br />
2. CrO 3<br />
A small amount <strong>of</strong> the isoprenyl<br />
isomer is also formed:<br />
Other disconnection approaches:<br />
O O<br />
Cl<br />
O<br />
or<br />
HO<br />
O<br />
AcOH, Ac 2O<br />
H 2SO 4<br />
For the - charge there is no easy synthon<br />
O<br />
MnO 2<br />
O
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
O O O<br />
Problem:<br />
1,4<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
Br<br />
Br<br />
1,2<br />
Enolate<br />
O<br />
1.2 or 1.4 attack, usually<br />
the 1.2 attack is the major<br />
one!<br />
Maybe it is possible to block the 2 Position, so that it would mainly be an attack from the 4-Position:<br />
SiMe 3<br />
O<br />
SiMe3 Problem:<br />
Brook rearrangement<br />
O<br />
Ways to get a Enone that is substituted in the para-Position:<br />
OTMS<br />
Method No.1:<br />
I OMe<br />
R (Nucleophile)<br />
+ Pd<br />
R<br />
OMe<br />
Li, NH3 (Birch)<br />
R<br />
OMe<br />
Method No.2:<br />
Use <strong>of</strong> the Danishefsky Diene<br />
OMe<br />
TMSO<br />
δ<br />
OMe<br />
δ<br />
R<br />
TMSO<br />
Treatment with H 3O + leads to the most<br />
stable conjugated enone.<br />
OMe<br />
OMe<br />
R<br />
H<br />
H 2O<br />
δ<br />
H<br />
H2O TMSO R Me R<br />
O R<br />
δ<br />
Problem: Due HOMO <strong>of</strong> the 2 Donors being on the same side the upper reaction will not work but will rather<br />
give the meta product. A possible solution would be to use a Dienophile with an EWG-group attached to it (e.g.<br />
an ester).<br />
O<br />
R<br />
R<br />
H 3O<br />
O
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
TMSO<br />
OMe<br />
δ<br />
δ<br />
CO 2R<br />
TMSO<br />
OMe<br />
Method 3: Micheal addition <strong>of</strong> an enamine.<br />
O<br />
R<br />
N<br />
H<br />
N<br />
R<br />
CO 2R<br />
<strong>Synthesis</strong> by Storck: JACS, 1973, 95, 3414<br />
O<br />
LDA<br />
O<br />
OEt O<br />
O<br />
N<br />
R<br />
H<br />
H 2O<br />
O<br />
2<br />
R<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
kinetic enolate<br />
O<br />
H<br />
thermodynamic enolate<br />
1<br />
N<br />
N<br />
R<br />
CO 2R<br />
Idea <strong>of</strong> Storck: Create the kinetic enolate and place something at the para-Position that can be turned later on<br />
into a ketone:<br />
O<br />
O<br />
O<br />
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong> by Storck<br />
O<br />
OEt<br />
Me<br />
O<br />
<strong>β</strong>-<strong>Vetivone</strong><br />
O<br />
H 3O<br />
OEt<br />
OEt<br />
LDA<br />
Me<br />
HO<br />
LDA<br />
O<br />
OEt<br />
O<br />
OEt<br />
kinetic enolate<br />
MeLi<br />
O<br />
2<br />
R<br />
R X<br />
OEt R<br />
OEt<br />
O<br />
Cl<br />
Cl<br />
OEt<br />
O<br />
O<br />
OEt<br />
6<br />
Cl<br />
O<br />
LDA<br />
HMPA<br />
O<br />
OEt<br />
Cl<br />
This Methyl group will<br />
direct the attack <strong>of</strong> the<br />
enolate.
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
<strong>Synthesis</strong> <strong>of</strong> starting material:<br />
O<br />
OEt<br />
OEt<br />
Cl<br />
Cl<br />
+<br />
O<br />
OH<br />
OH<br />
OEt<br />
OEt<br />
O<br />
O<br />
OEt<br />
OEt<br />
O<br />
O<br />
The last step didn't work due to following side reaction:<br />
The fast ring closure is due to the a very reactive LG (Allylic chloride)<br />
OH<br />
New approach:<br />
OH SO 2Cl<br />
OH 2 eq. n-BuLi<br />
OH<br />
O<br />
Cl<br />
OH<br />
LiAlH 4<br />
O 2 eq. MsCl<br />
O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
+<br />
O<br />
OH<br />
OH<br />
Reacts faster due to being<br />
the less stabilized alcoholate<br />
Yields are rather moderate but the dichloride could be obtained.<br />
Revisiting dipolar, aprotic solvents<br />
Me<br />
O<br />
S<br />
Me<br />
H<br />
O<br />
N Me<br />
Me<br />
DMSO DMF<br />
Most important ones are DMSO and DMF<br />
Cl<br />
Cl<br />
SO 2Cl<br />
O<br />
OMs<br />
HMPA<br />
LiCl<br />
Cl<br />
Cl<br />
The allylic alcoholate<br />
is not very reactive and<br />
give is therefore quickly<br />
Mesylated before<br />
undergoing ring -<br />
closure.<br />
OMs<br />
OMs<br />
HMPA makes the Cl -<br />
very nucleophilic.
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
HMPA Hexamethylphosphoramide<br />
HMPA<br />
Me 2N<br />
Me2N Me2N P O<br />
The most polar solvent that exists (Problem: might cause nasal cancers). HMPA is used as a solvent for<br />
polymers, gases, and organometallic reagents. It usefully improves the selectivity <strong>of</strong> lithiation reactions,<br />
because it breaks up the oligomers <strong>of</strong> lithium bases such as butyllithium. Because HMPA solvates cations so<br />
well, while not solvating anions, it accelerates some difficult SN2 reactions. The basic oxygen atom in HMPA<br />
coordinates strongly to Li + . Was used on a large scale as lubricant solvent in industry.<br />
DMPU<br />
DMPU<br />
O<br />
N N<br />
DMPU is a cyclic urea sometimes used as a polar, aprotic organic solvent. D. Seebach showed that it is possible<br />
to substitute the relatively toxic hexamethylphosphoramide (HMPA) with DMPU.<br />
<strong>Synthesis</strong> <strong>of</strong> Asaoka Chem. Lett. 1988, 1225<br />
Asaoka found a way to synthesize the enantiopure starting material (this will not be discussed here). The<br />
strategy <strong>of</strong> the synthesis is pretty much the same as the one used by Stork:<br />
Me 3Si<br />
O<br />
1) MeMgBr<br />
5% CuX<br />
2) TMS-Cl<br />
The 1.4 addition is directed<br />
mainly by the TMS-group<br />
so that a single diasteroisomer<br />
is formed.<br />
Me 3Si<br />
Analogous<br />
to Peterson<br />
Elimination<br />
Cl<br />
F<br />
O<br />
O<br />
Me<br />
TBAF<br />
(Bu4N + F- )<br />
Me<br />
Me 3Si<br />
MeLi<br />
1. LDA<br />
2. NCS<br />
OTMS<br />
Me<br />
Me 3Si<br />
OH<br />
MeLi<br />
Me<br />
O<br />
Me 3Si<br />
PCC<br />
Me<br />
OLi<br />
Me<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
Allylic bromide<br />
reacts faster than the<br />
primary alkyl-bromide<br />
attack from<br />
upper side<br />
Me<br />
<strong>β</strong>-<strong>Vetivone</strong><br />
Br<br />
Br<br />
Me 3Si<br />
The enolate here<br />
would lead to a 7membered<br />
cycle<br />
-Disfavored-<br />
NCS =<br />
Me 3Si<br />
7<br />
O<br />
O<br />
N-Chloro-succinimid<br />
Me<br />
EtO , Na<br />
EtOH<br />
Na<br />
1<br />
Me<br />
O<br />
O<br />
Br<br />
5<br />
Br<br />
N Cl
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
The last step <strong>of</strong> the oxidation reaction with PCC we have already seen in the synthesis <strong>of</strong> JASMONE, here a little<br />
reminder:<br />
O<br />
Thermodynamically more<br />
stable due to being<br />
a trisubstituted double<br />
bond.<br />
O<br />
MeLi<br />
Me<br />
Jasmone<br />
<strong>Synthesis</strong> <strong>of</strong> Posner JOC 1988, 53, 6031<br />
MeO<br />
O<br />
S<br />
O<br />
O<br />
MeTi(OiPr) 3<br />
THF<br />
Me CrO3, verd. H2SO4, Aceton<br />
OH<br />
Jones-Reagent<br />
CrO 3<br />
MeO<br />
HO<br />
Revisisiting: 1.4 Addition to chiral sulfoxides:<br />
1.4 Addition to chiral sulfoxides:<br />
µ O O<br />
µ Decreasing Dipole-<br />
R<br />
S<br />
Dipole interaction<br />
R<br />
O<br />
S<br />
R<br />
O<br />
Al (Hg)<br />
created by reaction<br />
<strong>of</strong> Al with HgCl 2<br />
Chirality transfer on Carbon<br />
OH<br />
R<br />
Al / Hg<br />
O<br />
S<br />
O<br />
S<br />
Zn 2<br />
O<br />
OH<br />
R<br />
R<br />
µ<br />
Hydride<br />
from above<br />
O<br />
R<br />
S<br />
O<br />
µ<br />
Cu-Organyl<br />
will attack from<br />
above!<br />
R<br />
DIBAL<br />
ZnCl 2<br />
O<br />
O<br />
S<br />
tertiary alcohol,<br />
H 2O is eliminated<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
tertiary alcohol cannot be oxidized!<br />
Me<br />
O<br />
S<br />
O<br />
Add metal<br />
M = Zn, Ti<br />
O<br />
R<br />
O<br />
DIBAL<br />
R<br />
Raney-Nickel<br />
EtOH<br />
M<br />
O O<br />
S<br />
Cu-Organyl<br />
will attack from<br />
below!<br />
(Clayden, Greeves, Warren and Wothers, p.1266, reprint 2004, Oxford University press<br />
O<br />
S<br />
Me<br />
Me<br />
O<br />
O<br />
51% yield<br />
93% ee<br />
Metal complexes<br />
the oxygens and<br />
attack leads to<br />
the other isomer!<br />
OH<br />
R<br />
Al / Hg<br />
O R<br />
S<br />
O<br />
Reduced<br />
Dipol-Dipol<br />
Interaction!<br />
Hydride<br />
from above<br />
OH<br />
R
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
Enolate can only<br />
be formed here<br />
O<br />
O<br />
This bond is the precursor<br />
<strong>of</strong> the C=O bond<br />
Methyl group is supposed to<br />
direct the reactions to give<br />
diasteromerically pure compounds.<br />
Posner is using the same dihalide precursor as Storck (see above)<br />
allylic bromide is<br />
O<br />
O<br />
substituted faster<br />
Br<br />
KHMDS<br />
Br<br />
O<br />
KHMDS<br />
+<br />
O<br />
Mesitoylchloride<br />
DMAP<br />
<strong>Synthesis</strong> <strong>of</strong> Dauben<br />
JACS 1975, 97, 1622<br />
JACS 1977, 99, 7307<br />
O<br />
O<br />
OH<br />
O<br />
Br<br />
OH<br />
1 eq.MeMgr<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
Use <strong>of</strong> Mesitoylchloride prrevents the ketone from being attacked again by MeMgBr<br />
to afford the corresponding tert-alcohol.<br />
O<br />
CrO 3 . 2Pyr<br />
Ph 3P CH 2<br />
Using the Fuchs (from Corey-Fuchs reaction) Synthon:<br />
O<br />
OH<br />
Br<br />
CH 2 O<br />
O<br />
1. CrO 3<br />
2. SnCl 4<br />
Oxidation to<br />
aldehyde and<br />
cyclisation<br />
(no standard<br />
chemistry)<br />
O<br />
O<br />
K<br />
O<br />
O<br />
LiAlH 4<br />
PPh3 BF4<br />
CO2Et - -<br />
The Counterion BF4 is chosen because Cl and other halides <strong>of</strong>ten give very hygroscopic Salts and are <strong>of</strong>ten less<br />
soluble.<br />
CH 2<br />
OH
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
Possible <strong>Synthesis</strong>:<br />
EtO<br />
Br<br />
O<br />
PPh 3<br />
Br 2<br />
For a simple system:<br />
EtO<br />
O<br />
PPh 3<br />
Br<br />
O O<br />
1. LDA<br />
2.<br />
O<br />
EtO H<br />
EtO<br />
O<br />
OEt<br />
Diethylcarbonate<br />
PPh 3<br />
EtO<br />
O<br />
Br<br />
O O<br />
H<br />
O<br />
CO 2Et<br />
EtO<br />
PPh 3<br />
O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
H<br />
CO 2Et<br />
O<br />
H<br />
PPh 3<br />
NaH<br />
Wittig<br />
base<br />
Br<br />
O<br />
Ph 3P<br />
O<br />
EtO<br />
EtO<br />
O<br />
O<br />
O<br />
Br<br />
O<br />
H<br />
PPh 3<br />
CO 2Et<br />
CO 2Et<br />
H<br />
PPh 3<br />
PPh 3
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
For the real system:<br />
EtO<br />
O<br />
1. Ac 2O<br />
2, BF 3<br />
by elimination<br />
<strong>of</strong> the tert-alcohol<br />
O<br />
1. NaH<br />
2.<br />
O<br />
EtO H<br />
EtO<br />
EtO<br />
<strong>Synthesis</strong> <strong>of</strong> Büchi JOC 1976, 41, 3208<br />
H<br />
H<br />
pKa = 15<br />
base<br />
O<br />
O<br />
OH<br />
O<br />
H<br />
MeLi<br />
NaH<br />
EtO<br />
This double bond formed<br />
due to being the most stable<br />
one (4 Subst instead <strong>of</strong> 3)<br />
H<br />
O<br />
R H<br />
EtO<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
O<br />
H<br />
Only this enantiomer shown,<br />
to explain diasteroselectivity<br />
<strong>of</strong> the reaction.<br />
O<br />
OH<br />
H<br />
R<br />
The Fuchs synthon comes in anti in regard to<br />
the methyl group.<br />
CO 2Et<br />
- H 2O<br />
PPh 3<br />
CO 2Et<br />
H 2 / Pd<br />
EtO<br />
EtO<br />
O<br />
Ph 3P<br />
O<br />
CO 2Et<br />
O<br />
Wittig<br />
H<br />
CO 2Et<br />
This double bond<br />
is more e - poor than the<br />
other one (even though the<br />
difference might not be that<br />
big)<br />
Fulvenes<br />
Fulvenes can react as a micheal acceptor. The driving force <strong>of</strong> the reaction is the creation <strong>of</strong> an aromatic<br />
system:<br />
Nu<br />
R<br />
For example: Nu = R 2CuLi (Cuprates)<br />
R<br />
Nu<br />
6 π-electron<br />
System<br />
R
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
<strong>Synthesis</strong> <strong>of</strong> the starting material:<br />
O<br />
5<br />
<strong>Synthesis</strong>:<br />
H<br />
H<br />
1<br />
O<br />
O<br />
Rationale:<br />
Pseudo 1,3 diaxial interaction<br />
O<br />
O O (Enolate <strong>of</strong> acetaldehye is very<br />
difficult to create, either the use<br />
<strong>of</strong> enamine chemistry or reacting<br />
n-BuLi with THF might help).<br />
O<br />
n-BuLi<br />
O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
Li<br />
+<br />
OLi<br />
This is the reason why reactions including THF and n-BuLi<br />
are <strong>of</strong>ten cooled down to -78°C.<br />
O<br />
O O<br />
favored<br />
(2 methyl are eq, ax)<br />
+<br />
disfavored<br />
(2 methyl are ax, ax)<br />
O<br />
Ozonolysis + work up with<br />
Me 2S<br />
O<br />
Me 2CuLi<br />
O<br />
1 single<br />
diasteroisomer<br />
is formed<br />
The hydrogenation <strong>of</strong> only on <strong>of</strong> the double bonds in the furane ring pro<strong>of</strong>ed to be very difficult. The only<br />
reagent found to do the job was Diimine:<br />
Reminder <strong>of</strong> diimine reduction<br />
Usually gives an excellent Z/E ration (up to 99.9%)<br />
<strong>Synthesis</strong> <strong>of</strong> H N N H :<br />
DEAD:<br />
EtO2C N N CO2Et H 2O / NaOH<br />
Na O2C N N CO2 Na<br />
Very reactive! H N N H<br />
HO2C N N CO<br />
- CO<br />
2H<br />
2<br />
Problem: Intermediates and the diimine might be explosive (Not done in industry, largest scale might be 2 or 3<br />
mmol).<br />
H<br />
OH
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
OH<br />
Might help to selectively<br />
reduce one double bond<br />
1) Ac 2O<br />
2) NH=NH (Diimine)<br />
Desymmetrizing the<br />
the 5-membered cycle<br />
Usual text-book chemistry <strong>of</strong> opening epoxides:<br />
O<br />
Nu<br />
Nu<br />
This might change quite a bit with sterically hindered nucleophiles:<br />
OH<br />
With Grignard reagents, X (e.g. Br) might turn out to be the nucleophile:<br />
O<br />
R Mg X<br />
With BuLi Deprotonation might occur:<br />
n-Bu Li<br />
O O<br />
This reaction was used by Büchi:<br />
OH<br />
O<br />
nBuLi<br />
X<br />
OH<br />
O<br />
OH<br />
The other H would<br />
be too hindered to<br />
deprotonate<br />
OH<br />
Example<br />
OH<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
O<br />
O<br />
O<br />
O H<br />
R Mg Br<br />
OH<br />
O<br />
OH<br />
O<br />
X<br />
PPh 3<br />
Wittig<br />
Br<br />
OH<br />
<strong>β</strong>−<strong>Vetivone</strong>