Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
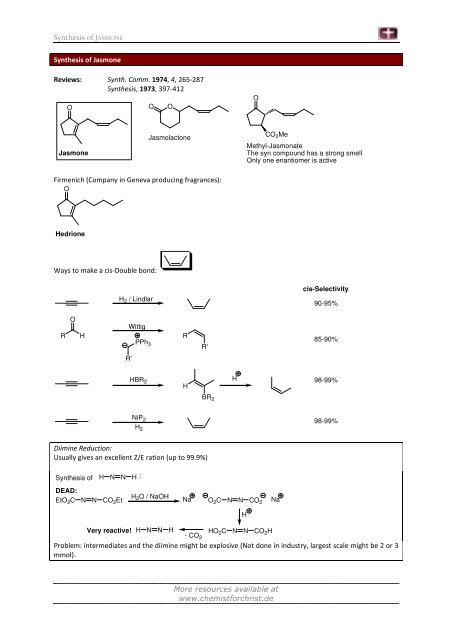
Synthesis of JASMONE<br />
Synthesis of <strong>Jasmone</strong><br />
Reviews: Synth. Comm. 1974, 4, 265-287<br />
Synthesis, 1973, 397-412<br />
O<br />
<strong>Jasmone</strong><br />
O<br />
O<br />
Jasmolactone<br />
Firmenich (Company in Geneva producing fragrances):<br />
O<br />
Hedrione<br />
Ways to make a cis-Double bond:<br />
O<br />
R H<br />
H 2 / Lindlar<br />
Wittig<br />
R'<br />
PPh 3<br />
HBR 2<br />
NiP 2<br />
H 2<br />
Diimine Reduction:<br />
Usually gives an excellent Z/E ration (up to 99.9%)<br />
Synthesis of H N N H :<br />
DEAD:<br />
EtO2C N N CO2Et H 2O / NaOH<br />
R<br />
H<br />
R'<br />
BR 2<br />
More resources available at<br />
www.chemistforchrist.de<br />
H<br />
O<br />
CO 2Me<br />
Na O2C N N CO2 Na<br />
H<br />
Methyl-Jasmonate<br />
The syn compound has a strong smell<br />
Only one enantiomer is active<br />
cis-Selectivity<br />
90-95%<br />
85-90%<br />
98-99%<br />
98-99%<br />
Very reactive! H N N H HO2C N N CO<br />
- CO<br />
2H<br />
2<br />
Problem: intermediates and the diimine might be explosive (Not done in industry, largest scale might be 2 or 3<br />
mmol).
Synthesis of JASMONE<br />
Phauson-Khand reaction: Synthesis of cyclopentenones via [2+2+1] cycloaddition<br />
Net-reaction:<br />
R L<br />
R S<br />
Mechanism:<br />
R S<br />
R S<br />
R<br />
R L<br />
R L<br />
R S<br />
O<br />
R<br />
Co(CO) 3<br />
Co(CO) 3<br />
R L<br />
Red. Elimination<br />
Co(CO) 3<br />
Co(CO) 3<br />
Co 2(CO) 8<br />
Co 2(CO) 8<br />
CO<br />
- 2 CO<br />
Insertion in<br />
red bond<br />
R S<br />
R L<br />
R S<br />
R S<br />
R<br />
O<br />
R<br />
O<br />
Review: Angew. Chem. Int. Ed. 2005, 44, 3022<br />
Aldolkondensation:<br />
O O<br />
OH<br />
R S<br />
R L<br />
R<br />
Co(CO) 3<br />
R L<br />
Co(CO) 3<br />
2<br />
3<br />
O<br />
1<br />
O<br />
R L<br />
CO comes from Co 2(CO) 8<br />
R<br />
Co(CO) 3<br />
Co<br />
(CO) 3<br />
Co(CO) 3<br />
Co(CO) 3<br />
4<br />
R migh also end up here,<br />
mixtures are formed.<br />
-[Co 2(CO) 6]<br />
More resources available at<br />
www.chemistforchrist.de<br />
R<br />
Insertion in<br />
red bond<br />
+ CO<br />
O<br />
1,4 Dicarbonyl compound<br />
R S<br />
R<br />
R S<br />
R S<br />
R L<br />
(OC) 2Co<br />
R L<br />
O<br />
Co(CO) 3<br />
Co<br />
(CO) To create a<br />
2<br />
free coordination<br />
site<br />
R<br />
R L<br />
Co(CO) 3<br />
R
Synthesis of JASMONE<br />
O<br />
O<br />
O<br />
O<br />
R 1<br />
R 1<br />
1<br />
2<br />
O<br />
O<br />
O<br />
O<br />
R 1<br />
R 1<br />
R 2<br />
R 2<br />
S S +<br />
Synthesis by S. Mann Synth. Comm. 1985, 15, 1147-1151<br />
O<br />
O<br />
O N<br />
O O Li<br />
Solution: Hydrazone<br />
O H 2N NMe 2<br />
O<br />
O<br />
O<br />
R 2<br />
made out of:<br />
O<br />
+<br />
N<br />
H<br />
N NMe 2<br />
n-Bu Li<br />
Cl<br />
O<br />
R 1<br />
XMg<br />
O<br />
More resources available at<br />
www.chemistforchrist.de<br />
+<br />
Nu<br />
Br<br />
R 2<br />
O O R2<br />
O O R2<br />
Br R 2<br />
Enamine is a too weak nucleophile.<br />
O<br />
To weak as a nucleophile?!<br />
N NMe 2<br />
Li<br />
O<br />
R 2<br />
O O R2<br />
very strong nucleophile<br />
(charge is not delocalized)
Synthesis of JASMONE<br />
Chiral Hydrazones: Enders<br />
N N<br />
OMe<br />
Is made out of proline<br />
by reduction and protection<br />
with MeI<br />
1<br />
2<br />
3<br />
O<br />
1<br />
2<br />
O<br />
O<br />
3<br />
N OMe<br />
Me<br />
Weinreb-Amid<br />
1.) n-BuLi<br />
2.) E<br />
3.) H 3O , CuSO 4<br />
+ X<br />
Wittig-Reaction<br />
O<br />
O<br />
X<br />
O<br />
+<br />
+<br />
O<br />
XMg<br />
+<br />
O<br />
H 2 , Lindlar-Pd<br />
PPh 3<br />
Aledehyde reacts faster than the ketone!<br />
O<br />
Commercially available<br />
E<br />
*<br />
SAMP RAMP<br />
N<br />
H<br />
O Mg<br />
N O<br />
More resources available at<br />
www.chemistforchrist.de<br />
R<br />
Problem:<br />
E/Z Isomers<br />
S or R<br />
OMe Amino<br />
Methoxy<br />
Pyrolidine
Synthesis of JASMONE<br />
Synthesis:<br />
EtO<br />
O<br />
O<br />
O<br />
O<br />
O<br />
1<br />
2<br />
H 2N NMe 2<br />
O<br />
100%<br />
90%<br />
+<br />
N NMe 2<br />
O<br />
10%<br />
Enolate formed at Position: 2 1<br />
electron poor due<br />
to conjugation<br />
R<br />
R<br />
O O<br />
E 2<br />
O<br />
E 1<br />
:<br />
1. O 3<br />
2. Me 2S<br />
O<br />
O<br />
Ph 3P<br />
Sterically hindered Base<br />
(Kinetically controlled deprotonation)<br />
NaOH<br />
to create the<br />
Ylide<br />
: Thermodynamically controlled deprotonation<br />
R<br />
R<br />
For E 1 = Br<br />
For E 2 =<br />
Br<br />
BH 4<br />
H 3O<br />
- CO 2<br />
O<br />
EtO<br />
EtO<br />
O O<br />
O O<br />
E 2<br />
R<br />
E 1<br />
R<br />
n-Bu Li<br />
More resources available at<br />
www.chemistforchrist.de<br />
Li<br />
E 2<br />
1. n-BuLi<br />
2.<br />
O<br />
3. H2O + Cu(OAc) 2<br />
O<br />
75%<br />
SiMe3 N<br />
SiMe3 EtO<br />
EtO<br />
E 1<br />
O O<br />
O O<br />
NaOH<br />
70%<br />
44% yield<br />
LMDS<br />
R<br />
O<br />
OH<br />
O<br />
O<br />
What was deprotonated<br />
last will react first!<br />
E 1<br />
R<br />
Oxidation<br />
with PCC
Synthesis of JASMONE<br />
Synthesis of Larry Weiler Can. J. Chem. 1978, 56, 2301-2304<br />
O<br />
O<br />
OEt<br />
1. Base<br />
2.<br />
O<br />
O CO2Et<br />
O<br />
<strong>Jasmone</strong><br />
Br<br />
NaOH<br />
Synthesis of Welch JOC 1987, 52, 1440-1450<br />
O<br />
O<br />
CO 2Et<br />
1. NaH<br />
2. n-BuLi<br />
3.<br />
O P(OEt) O<br />
2<br />
Cl<br />
CO 2Et<br />
O P(OEt)2<br />
O<br />
NaOH<br />
O<br />
Cl<br />
O<br />
H 3O<br />
HgSO 4<br />
O<br />
O<br />
+<br />
OEt<br />
CO 2Et<br />
<strong>Jasmone</strong><br />
1. NaH<br />
2. n-BuLi<br />
3.<br />
Br<br />
O CO2Et<br />
More resources available at<br />
www.chemistforchrist.de<br />
H<br />
HgSO 4<br />
R H 2SO 4<br />
TMS<br />
KF<br />
R<br />
O O<br />
Lindlar / Pd<br />
OH<br />
TMS<br />
O CO2Et<br />
TMS<br />
OEt<br />
R<br />
Steric bulk<br />
hinders<br />
Hydrogenation<br />
O
Synthesis of JASMONE<br />
Additional information:<br />
Reaction of Perkow: JACS 1955, 77, 2871<br />
Perkow:<br />
O<br />
O<br />
Cl Cl<br />
O<br />
Cl Cl<br />
Arbusow:<br />
EtO<br />
EtO P<br />
EtO<br />
P(OEt) 3<br />
∆<br />
P(OEt) 3<br />
R Br<br />
Cl<br />
Cl<br />
O<br />
O<br />
EtO<br />
EtO P<br />
O<br />
P(OEt) 2<br />
P(OEt) 3<br />
Et<br />
Br<br />
HWE-Reagent (HWE = Horner-Wadsworth-Emmons)<br />
Synthesis by McMurry<br />
JOCS 1973, 38, 4367-4373<br />
JACS 1971, 93 5309-5311<br />
New approach to create the 1,4 diketone<br />
2<br />
3<br />
O<br />
1<br />
4<br />
O<br />
O<br />
+<br />
R<br />
O<br />
R<br />
R<br />
S S<br />
NO 2<br />
The conditions of the Nef-reaction are rather harsh:<br />
O O<br />
N<br />
H<br />
OH<br />
NaOH<br />
O O<br />
N<br />
H 2SO 4<br />
conc.<br />
reflux<br />
R<br />
R<br />
Cl<br />
Cl<br />
HO OH<br />
N<br />
O<br />
O<br />
O<br />
EtO<br />
EtO P<br />
O<br />
P(OEt) 2<br />
P(OEt) 2<br />
Et<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
H 2O<br />
R<br />
+ EtBr<br />
S S<br />
E<br />
E<br />
NO 2<br />
R<br />
R<br />
O<br />
Hg(II)<br />
1. NaOH<br />
2. H 2SO 4<br />
Nef-reaction<br />
Et<br />
Et<br />
O<br />
O<br />
R<br />
R
Synthesis of JASMONE<br />
McMurrys attention was therefore drawn to a publication of Wildsmith, who used much milder conditions to<br />
hydrolyze an oxime (which is not that far away from a nitro group):<br />
Synthesis by Wildsmith TL 1971, 195-198<br />
Utilisisation of TiCl3 for the Nef reaction: Tetrahedron Letters, 1971, 195-198<br />
N OH<br />
NH<br />
1.<br />
2. H<br />
O<br />
2O<br />
Oxime<br />
TiCl 3<br />
McMurry was lucky, the reaction worked:<br />
O O<br />
N<br />
nitro<br />
Synthesis:<br />
H<br />
NO 2<br />
NO 2<br />
TiCl 3<br />
base<br />
OH<br />
N N O<br />
H<br />
oxime imine<br />
NaNO 2<br />
NH<br />
O<br />
I<br />
2 eq.<br />
NO 2<br />
O<br />
HO Br<br />
HI<br />
or:<br />
1. TsCl<br />
2. I<br />
More resources available at<br />
www.chemistforchrist.de<br />
HO<br />
HO<br />
TiCl 3<br />
diglyme, ∆<br />
MeO O OMe<br />
Lindlar Pd<br />
O<br />
O<br />
O<br />
<strong>Jasmone</strong><br />
NaOH
Synthesis of JASMONE<br />
Synthesis by Givaudan, Helv. Chim. Acta 1978, 61, 990-997<br />
Not a very good Electrophile<br />
due to having a quite acidic<br />
Proton in an allylic Position<br />
Nu<br />
That's why he decided rather to use it as a nucleophile:<br />
That is present in a certain<br />
fraction in petrol refinery<br />
(The other compounds present<br />
in the mixture are usually<br />
hydrocarbons and do therefore<br />
not react - pure Butin is very expensive)<br />
O<br />
<strong>Jasmone</strong><br />
n-Bu Li Li<br />
O<br />
OH<br />
NaOH<br />
JONES-OX<br />
H<br />
MgBr<br />
O<br />
Br<br />
Jones Reagent:<br />
CrO 3, diluted H 2SO 4, acetone<br />
Mg<br />
Nef-reaction<br />
Synthesis of <strong>Jasmone</strong> by Stetter, Synthesis 1975, 379-380<br />
d 1<br />
O<br />
Might be:<br />
NO 2<br />
1<br />
O<br />
2<br />
OR<br />
CN<br />
3<br />
O<br />
4<br />
EtO<br />
S<br />
undesired<br />
side reaction<br />
O<br />
More resources available at<br />
www.chemistforchrist.de<br />
S<br />
R<br />
MgBr<br />
OH<br />
Lindlar-Pd,<br />
H 2<br />
HBr<br />
NaOH<br />
OH<br />
Br<br />
NO 2<br />
O<br />
Conj. Addition<br />
NO 2
Synthesis of JASMONE<br />
R<br />
O<br />
Ph H<br />
Ph H<br />
O<br />
O<br />
H<br />
+ cat. KCN<br />
+<br />
CN<br />
In the presence of:<br />
cat.<br />
CN<br />
R<br />
O<br />
CN<br />
O<br />
Ph H<br />
CN<br />
H<br />
CN<br />
Ph<br />
+ Ph<br />
O<br />
O<br />
R<br />
OH<br />
Ph<br />
Ph<br />
OH<br />
OH<br />
CN<br />
Ph<br />
OH<br />
CN<br />
O Will react much slower with CN , because the<br />
Carbonylfunction is stabilized by conjugation.<br />
Stetter: Biomimetic Reaction<br />
Tetrahedron Letters, 1974, 4505-4508<br />
Review: Org. React. 1991, 40, 407-496<br />
Biomimetic: Means copied from nature.<br />
Vitamine B1 (Thiamine)<br />
thiamine<br />
HO<br />
Cl -<br />
N +<br />
S<br />
HO<br />
NH 2<br />
N<br />
N<br />
N<br />
S<br />
Bn<br />
O<br />
R 1<br />
benzoine<br />
Ph<br />
Ph<br />
O<br />
Conj. Add.<br />
More resources available at<br />
www.chemistforchrist.de<br />
2<br />
3<br />
4 Me<br />
O<br />
Simplified by Stetter:<br />
HO<br />
S<br />
Thiazolium-Salt<br />
HO<br />
X<br />
N +<br />
H<br />
Ph<br />
NaOH<br />
N<br />
S<br />
Bn<br />
OH<br />
CN<br />
O<br />
Ph<br />
more acidic<br />
as the other<br />
O<br />
CN<br />
R<br />
R<br />
OH<br />
OH<br />
CN<br />
O<br />
CN<br />
Ph<br />
O<br />
O<br />
Me<br />
Me<br />
Today also chiral versions<br />
for asymmetric reactions.<br />
This proton is quite acidic!<br />
This anion replaces the role of the CN that we saw in the reaction above.
Synthesis of JASMONE<br />
Another way to see it is:<br />
Bn<br />
HO<br />
N<br />
S<br />
A nucleophilic Carbene<br />
This kind of carbenes are pretty important today:<br />
Ar The first carbene that was isolated, crystalized and characterized.<br />
N Today there are many chiral versions available that are used<br />
in asymmetric synthesis.<br />
N<br />
Ar<br />
N<br />
N<br />
Ar<br />
Ar<br />
Very reactive<br />
species! Prone<br />
to nucleophilic<br />
attack.<br />
N<br />
N<br />
Nu<br />
H<br />
N<br />
N<br />
N<br />
N<br />
Ar<br />
Ar<br />
6-π Electron aromatic<br />
system, that's why the carbene<br />
is so stable!<br />
Idea:<br />
Block the site prone<br />
to nucleophilic attack<br />
with very bulky substituents.<br />
The 2 plane where the 2 mesityl<br />
substituents are lying in are<br />
perpendicular to each other.<br />
More resources available at<br />
www.chemistforchrist.de<br />
Putting different charges on the<br />
same carbon atom can also be a<br />
way of representing a carbene!<br />
N<br />
N<br />
N<br />
N<br />
Mesityl- Adamantyl-<br />
The fact that carbenes can be used in catalysis as a Ligand is due to the fact that the 2 Electrons in the sp 2<br />
Orbital behave like lone pair and can thus donate electron density to the corresponding metal.<br />
R<br />
N<br />
N<br />
R<br />
behaves like a<br />
Donor e.g.<br />
R<br />
R P<br />
R<br />
Carbenes in catalysis are often named NHC-Ligands (N-Heterocyclic Carbenes) and offer many advantages<br />
compare to their Phosphorous counterparts:<br />
a) Environmentally more friendly (often non toxic)<br />
b) Not sensitive to oxidation<br />
c) Lower Molecular mass, for industrial purpose this means less gramms or kg of Ligand has to be added.
Synthesis of JASMONE<br />
O<br />
O<br />
O<br />
(with cat. Stetter reagent)<br />
+<br />
O<br />
BrMg<br />
Br<br />
HO<br />
More resources available at<br />
www.chemistforchrist.de<br />
+ DMF<br />
(already discussed<br />
see above)<br />
For adding a formyl DMF is the best reagent (Adding the Weinrebamide would more complicated).<br />
O<br />
H OH<br />
O<br />
H OH<br />
SO 2Cl 2<br />
DCC<br />
Me<br />
HN<br />
OMe<br />
O<br />
H Cl<br />
H<br />
O<br />
N<br />
Me<br />
OMe<br />
instable<br />
BrMg<br />
CO + HCl<br />
Historically Formyl groups were added with the help of Orthoesters:<br />
Et Et<br />
O<br />
R MgX<br />
Et O in the presence of :<br />
Heat > 35°C<br />
and evaporate<br />
R MgX<br />
Et<br />
OEt<br />
HC OEt<br />
OEt<br />
Orthoester<br />
the ether<br />
OEt<br />
HC OEt<br />
OEt<br />
Stetter chose to change the orthoester in making<br />
one of the OEt group to a better leaving group a<br />
Phenol (OPh). This facilitates the reaction and it can<br />
be done now at r.t.<br />
OEt<br />
HC OEt<br />
OEt<br />
+ PhOH HC<br />
OEt<br />
OEt<br />
OPh<br />
+ HOEt<br />
Utilisation of mixed othoformiates: Chem. Ber. 1970, 103, 643<br />
Synthesis of Stetter<br />
MgBr<br />
OEt<br />
HC OEt<br />
OPh<br />
O<br />
<strong>Jasmone</strong><br />
R<br />
O<br />
H<br />
H<br />
H 3O<br />
O<br />
R<br />
H<br />
OEt<br />
OEt<br />
Highly reactive,<br />
will react immediately<br />
with the grignard<br />
reagent.<br />
R<br />
R MgX<br />
OEt<br />
H<br />
OEt<br />
OEt O<br />
H3O OEt<br />
NaOH<br />
10 mol%<br />
Thiazolium-Salt<br />
O<br />
4<br />
3<br />
O<br />
2<br />
1<br />
O
Synthesis of JASMONE<br />
Different approach using Photo-reactions:<br />
O<br />
rotation of<br />
C-C bond<br />
O<br />
O<br />
HIO4 OH OH<br />
R<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Problem:<br />
hν<br />
hν<br />
OH BrMg<br />
O<br />
O OH<br />
5<br />
4<br />
H<br />
3<br />
O<br />
1<br />
O<br />
2<br />
Biradikal<br />
Does it come from above<br />
or below?<br />
Answer: 50:50<br />
1,5 H-Shift<br />
Synthesis by Büchi JOC 1966, 31, 977-978<br />
O<br />
Molecules with the same oxidation state:<br />
N<br />
Imine<br />
Synthesis in 3 steps<br />
O<br />
O<br />
O<br />
O<br />
Acetale<br />
H<br />
N<br />
NH<br />
Aminale<br />
OH<br />
R<br />
OH<br />
OH<br />
OH<br />
R<br />
OH<br />
O O<br />
50 : 50<br />
Only this diastereoisomer<br />
will react with HIO 4, the other<br />
cannot react in a syn-fashion.<br />
More resources available at<br />
www.chemistforchrist.de<br />
N<br />
Enamine<br />
R<br />
O<br />
R<br />
O<br />
OsO 4<br />
R<br />
very difficult<br />
to synthesize<br />
OH<br />
OH<br />
OH<br />
R<br />
Enole / Enole-ether<br />
Why not putting the 2 OH<br />
groups together?<br />
To hydrolyze a furane is not that easy because it is aromatic, but it can be done!
Synthesis of JASMONE<br />
one step<br />
O<br />
n-Bu Li<br />
O<br />
O<br />
Li<br />
<strong>Jasmone</strong><br />
Br<br />
As discussed earlier not<br />
a good E but used anyway<br />
40%<br />
(3 steps)<br />
NaOH<br />
Synthesis of <strong>Jasmone</strong> by Hiyama, Bull. Chem. Soc. Jpn. 1980, 53, 169-173<br />
(Electrocylic reactions)<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
1<br />
O<br />
2<br />
3<br />
AcOH,<br />
H 2SO 4<br />
H 2O<br />
O<br />
4<br />
Problem: under the very acidic<br />
hydrolysis condition E/Z isomerization<br />
takes place! - 7% E Isomer is yielded<br />
In an electrocyclic reaction a ring is always broken or formed. Rings may, of course be formed by cycloadditions<br />
as well, but the difference with electrocyclic reactions is that just one new σ bond is formed (or broken) across<br />
the ends of a single conjugated π system.<br />
The types of pericyclic reactions are distinguished by the number of σ bonds made or broken.<br />
Cycloadditions<br />
2 new σ-bonds are formed<br />
... or broken<br />
∆σ = 2<br />
Types of pericyclic reactions<br />
Sigmatropic<br />
rearrangements<br />
One new σ-bonds is<br />
formed as another is<br />
broken.<br />
∆σ = 0<br />
Electrocyclic reactions<br />
One new σ-bonds is formed<br />
... or broken<br />
∆σ = 1<br />
Rules for electrocyclic reactions<br />
All electrocyclic reactions are allowed<br />
Thermal electrocyclic reactions involving (4n+2)π electrons are disrotatory:<br />
one group rotates clockwise and one anticlockwise<br />
Thermal electrocyclic reactions involving (4n)π electrons are conrotatory, in contrary reactions the two groups<br />
rotate in the same way:<br />
both clockwise both counterclockwise
Synthesis of JASMONE<br />
The Nazarow reaction: Short revision<br />
For more details see: Org. React. 1994, 45, 1-158<br />
O<br />
O<br />
R R<br />
O<br />
H<br />
hν (254 nm)<br />
benzene<br />
AcOH, H 3PO 4<br />
50°C<br />
R R<br />
Disrotatory Conrotatory<br />
O<br />
R R<br />
R R<br />
In its simplest form the Nazarow cyclization is the ring closure of a doubly α,β unsaturated ketone to give a<br />
cyclopentenone.<br />
O<br />
H<br />
R R<br />
O<br />
H<br />
R R<br />
R<br />
empty<br />
OH<br />
More resources available at<br />
www.chemistforchrist.de<br />
R<br />
O<br />
R R<br />
array of five p orbitals<br />
containing 4p electrons<br />
One of the five π orbitals involved is empty, so the cyclization is a 4π electrocyclic reaction and the orbitals<br />
forming the new σ bond must interact antarafacially.<br />
H<br />
O<br />
OH<br />
O<br />
OH<br />
OH<br />
R R<br />
R<br />
π 4<br />
a<br />
R<br />
H<br />
R<br />
R<br />
R R<br />
R R<br />
cyclopentenone<br />
Usually the double bond is formed at the site which is higher substituted (thermodynamically more stable<br />
product) to get the double bond at the less substituted site can also be done by introducing a siliconsubstituent:<br />
O<br />
TMS R<br />
Lewis acid<br />
LA<br />
O<br />
TMS R<br />
Nazarow<br />
LA<br />
O<br />
TMS R<br />
Anion<br />
H 3O<br />
O<br />
R
Synthesis of JASMONE<br />
Synthesis of Hiyama Bull. Chem. Soc. Jpn 1980, 53, 169-173<br />
O<br />
OH<br />
O<br />
HCOOH,<br />
H 3PO 4<br />
Difficult mechanism:<br />
Tautomerization,<br />
Isomerization,<br />
Eliminatation....<br />
For more details consult<br />
literature given.<br />
Some commercially available 5-memebered rings:<br />
O O O<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
is like other 1,3 diketones<br />
in equilibrium with its<br />
enol.<br />
O H<br />
OH<br />
H<br />
O<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
O H<br />
best nucleophile<br />
in the mixture<br />
cyclopentane-1,3-dione<br />
Is an important starting material<br />
for the industrial synthesis of<br />
steroids.<br />
O O<br />
The Keto-Enol Tautomerization can be seen in the 1 H-NMR.<br />
O<br />
Examples for E :<br />
O<br />
soft E<br />
E<br />
R I<br />
OH<br />
hard E<br />
Vinylogous Behaviour:<br />
O<br />
OH<br />
behaves like a<br />
carboxlic acid<br />
pK a = 9-10<br />
O<br />
OH<br />
O<br />
OH<br />
E<br />
O<br />
,<br />
EtO Cl<br />
Synthesis by Piers et al Can. J. Chem. 1982, 60, 1256-1263<br />
O O<br />
O<br />
R<br />
O<br />
O<br />
This one also<br />
O<br />
O<br />
O<br />
OH<br />
H<br />
O O
Synthesis of JASMONE<br />
O<br />
O<br />
NaOH<br />
Even easier:<br />
O<br />
O<br />
O<br />
O<br />
Cl<br />
soft E<br />
O<br />
Na O<br />
EtOH, H<br />
(due to Vinologous<br />
behaviour same<br />
conditions can be<br />
used as for Carboxylic<br />
acids!)<br />
O<br />
OEt<br />
Me<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
Br 2, PPh 3<br />
(due to Vinologous<br />
behaviour same<br />
conditions can be<br />
used as for Carboxylic<br />
acids!)<br />
MeLi<br />
This is a electron rich<br />
double bond and therefore<br />
Me 2CuLi doesn't react nicely<br />
with this molecule.<br />
Review on conjugated addition of Organocopper reagents: Org. React. 1972, 19, 1-113<br />
Org. React. 1992, 42, 135-631<br />
Revisit on Organocopper reagents:<br />
Homocuprates (Gilman reagents): Me2CuLi, Bu2CuLi<br />
e.g. Me2CuLi, Bu2CuLi<br />
Mixed homocuprate: RCuR'Li<br />
Mixed homocuprates are used in order not to waste halt of the Substituent R:<br />
R MgX + CuCl 2 R Cu + RCl + MgXCl<br />
MeCuBuLi (Bu-Substituent is transfered),<br />
transfered!)<br />
R<br />
Li<br />
Cu R'<br />
Heterocuprates: RCuXR'<br />
e.g. -SPh, -OtBu (these substituents are never transfered)<br />
R Li + CuSPh RCuSPhLi<br />
Me<br />
Me<br />
OH<br />
OEt<br />
O<br />
O<br />
O<br />
Me<br />
Br<br />
Br<br />
H<br />
Me 2CuLi<br />
(The R substituent is transfered, alkynes are never<br />
R3CuLi2 (higher order cuprates)<br />
Those were discovered by following experiment: MeLi and CuI were mixed in various rations and the Me-<br />
Singlet in the 1 H-NMR was observed: When mixing MeLi and CuI in a 3:1 ratio only a single species could be<br />
seen.<br />
Cyanocuprates (Lipshutz cuprates) R3CuCNLi2<br />
2 RLi + CuCN R 2CuCNLi 2 (R 2CuLi, LiCN)<br />
Cyanide (CN) acts as the 3rd R group in relation to the creation of higher order cuprates.
Synthesis of JASMONE<br />
The LiI that is created while reacting RLi and CuI is absolutely necessary for reactions to take place!<br />
The cuprates have a shape of a plane square, therefore reactions can done highly<br />
diastereoseletive because cuprates are quite sensitive to sterical effects:<br />
Cuprate<br />
O<br />
Example for an application of organocopper reagents: 1,4 Addition to Pyridinium-Salts<br />
R'<br />
N<br />
R'<br />
O<br />
R Cl<br />
N<br />
R O<br />
R'<br />
RMgX<br />
R 2CuLi<br />
More resources available at<br />
www.chemistforchrist.de<br />
N<br />
R O<br />
R O<br />
Construction of molecules that resemble natures NAD, NADH<br />
Synthesis of Grieco JOC, 1972, 37, 2363-2364<br />
O<br />
C<br />
Cl Cl<br />
Made out of:<br />
, H<br />
Heating and destillation<br />
of cyclopentadiene<br />
O<br />
C<br />
Cl Cl<br />
O<br />
Cl<br />
Cl<br />
[2+2]<br />
[2+2]<br />
Bayer Villinger<br />
AcOH, H 2O 2<br />
CH 2N 2<br />
O<br />
C<br />
Cl Cl<br />
stable<br />
O<br />
C<br />
H H<br />
O<br />
Cl<br />
Cl<br />
O<br />
Cl<br />
O<br />
R<br />
*<br />
N<br />
*<br />
Made out of:<br />
Not stable,<br />
reacts with<br />
itself:<br />
Cl<br />
Cl<br />
O<br />
O<br />
O<br />
R<br />
R'<br />
Cl<br />
Cl Cl + Zn or<br />
Cl<br />
O<br />
O<br />
O<br />
Cl<br />
Cl H NEt 3
Synthesis of JASMONE<br />
O<br />
O<br />
DIBAL-H<br />
For a lactone the best<br />
reducing agent to a lactole<br />
is DIBAL-H, for an ester this<br />
might not work so well!<br />
H<br />
OH<br />
O<br />
Thermodynamically more<br />
stable due to being<br />
a trisubstituted double<br />
bond.<br />
O<br />
under the acidc<br />
conditions:<br />
isomerization<br />
MeLi<br />
Me<br />
<strong>Jasmone</strong><br />
O<br />
O<br />
OH<br />
More resources available at<br />
www.chemistforchrist.de<br />
OH<br />
O<br />
CrO 3, verd. H 2SO 4, Aceton<br />
Jones-Reagent<br />
Me CrO3, verd. H2SO4, Aceton<br />
OH<br />
Jones-Reagent<br />
CrO 3<br />
HO<br />
tertiary alcohol,<br />
H 2O is eliminated<br />
Wittig<br />
tertiary alcohol cannot be oxidized!<br />
The textbook Clayden, Greeves, Warren and Wothers (Oxford University press, p. 951, 2004 reprint) proposes<br />
a different mechanism:<br />
OH (VI)<br />
OH<br />
O<br />
R Li<br />
R<br />
O<br />
CrO3 R (VI)<br />
CrO<br />
orange<br />
3<br />
OH<br />
R CrO 3<br />
H<br />
O OH<br />
Cr<br />
O O<br />
R<br />
H<br />
[3,3]<br />
HO OH<br />
Cr<br />
O O<br />
H<br />
H R<br />
Me<br />
O<br />
(III)<br />
CrO3 R<br />
+ Cr(IV)<br />
green<br />
PPh 3<br />
OH<br />
Me<br />
Me<br />
gives Cr(III) and Cr(VI)<br />
by disproportionation.<br />
The first step is the formation of a chromate ester but this intermediate has no proton to lose, so it transfers<br />
the chromate to the other end of the allylic system where there is a proton. The chromate transfer can be<br />
drawn as a [3,3] sigmatropic rearrangement.<br />
Very general method:<br />
R' MgBr<br />
O<br />
R<br />
HO<br />
R'<br />
R'<br />
R H<br />
R<br />
OH<br />
Ox<br />
R'<br />
R<br />
O
Synthesis of JASMONE<br />
Cope Rearrangement<br />
1 2<br />
1<br />
2<br />
3<br />
4<br />
5<br />
Cope<br />
2<br />
1 2<br />
1<br />
3 4<br />
Synthesis of Descotes Synthesis 1975, 118-119<br />
3<br />
O<br />
3<br />
4<br />
2 6<br />
H<br />
1<br />
5<br />
4<br />
2 6<br />
H<br />
1<br />
CO 2Et<br />
CO 2Et<br />
5<br />
much<br />
cheaper<br />
5<br />
H<br />
Z<br />
Cope<br />
O O<br />
YZn X<br />
CH2<br />
EtO 2C CO 2Et<br />
CO 2Et<br />
EtO 2C CO 2Et K 2CO 3 EtO 2C CO 2Et Br<br />
Br<br />
+<br />
Br<br />
EtO 2C CO 2Et<br />
2 steps as<br />
explained above<br />
(Grignard, Oxidation)<br />
CO 2Et<br />
+ K 2CO 3<br />
intramolecular<br />
reaction<br />
(ring closure)<br />
More resources available at<br />
www.chemistforchrist.de<br />
Simmons Smith<br />
Br EtO 2C CO 2Et<br />
Technique to enlarge<br />
cyclic systems<br />
Br<br />
<strong>Jasmone</strong><br />
K 2CO 3<br />
EtO 2C CO 2Et<br />
Br
Synthesis of JASMONE<br />
Br<br />
Br<br />
Br<br />
+<br />
CO 2Et<br />
CO 2Et<br />
CO2Et CO2Et S N2'<br />
K2CO3 CO2Et ?<br />
DMF CO2Et CO 2Et<br />
CO 2Et<br />
∆<br />
150°C<br />
CO2Et ?<br />
CO2Et Industrial Synthesis (Firmenich) Helv. Chim. Acta 1978, 61, 2524-2529<br />
O<br />
+ Br 2 (hν)<br />
(heat in a radical<br />
fashion)<br />
Br<br />
Br<br />
+<br />
Br<br />
Br<br />
Br<br />
Br<br />
E/Z mixture<br />
and..<br />
+<br />
Br<br />
Br<br />
via the<br />
enolate<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
O<br />
> 150°C<br />
The Problem is that during the radical bromination epoxides are involved due to the presence of air and one<br />
day the plant that produced <strong>Jasmone</strong> via this pathway was blown up and since then this reaction is not used<br />
industrially anymore.