Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
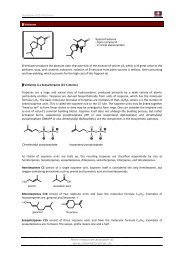
Synthesis of JASMONE<br />
Synthesis of Hiyama Bull. Chem. Soc. Jpn 1980, 53, 169-173<br />
O<br />
OH<br />
O<br />
HCOOH,<br />
H 3PO 4<br />
Difficult mechanism:<br />
Tautomerization,<br />
Isomerization,<br />
Eliminatation....<br />
For more details consult<br />
literature given.<br />
Some commercially available 5-memebered rings:<br />
O O O<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
is like other 1,3 diketones<br />
in equilibrium with its<br />
enol.<br />
O H<br />
OH<br />
H<br />
O<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
O H<br />
best nucleophile<br />
in the mixture<br />
cyclopentane-1,3-dione<br />
Is an important starting material<br />
for the industrial synthesis of<br />
steroids.<br />
O O<br />
The Keto-Enol Tautomerization can be seen in the 1 H-NMR.<br />
O<br />
Examples for E :<br />
O<br />
soft E<br />
E<br />
R I<br />
OH<br />
hard E<br />
Vinylogous Behaviour:<br />
O<br />
OH<br />
behaves like a<br />
carboxlic acid<br />
pK a = 9-10<br />
O<br />
OH<br />
O<br />
OH<br />
E<br />
O<br />
,<br />
EtO Cl<br />
Synthesis by Piers et al Can. J. Chem. 1982, 60, 1256-1263<br />
O O<br />
O<br />
R<br />
O<br />
O<br />
This one also<br />
O<br />
O<br />
O<br />
OH<br />
H<br />
O O