Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
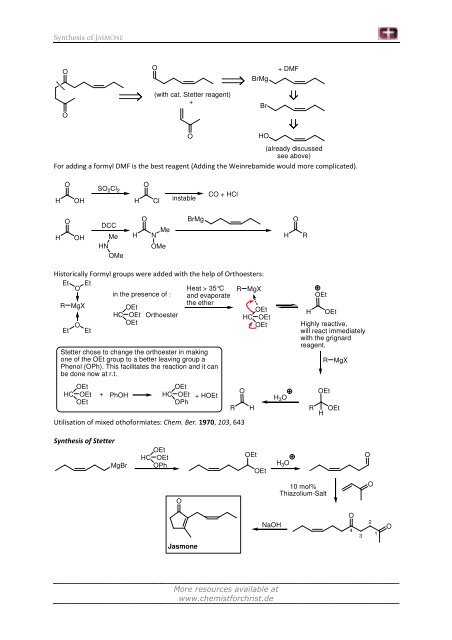
Synthesis of JASMONE<br />
O<br />
O<br />
O<br />
(with cat. Stetter reagent)<br />
+<br />
O<br />
BrMg<br />
Br<br />
HO<br />
More resources available at<br />
www.chemistforchrist.de<br />
+ DMF<br />
(already discussed<br />
see above)<br />
For adding a formyl DMF is the best reagent (Adding the Weinrebamide would more complicated).<br />
O<br />
H OH<br />
O<br />
H OH<br />
SO 2Cl 2<br />
DCC<br />
Me<br />
HN<br />
OMe<br />
O<br />
H Cl<br />
H<br />
O<br />
N<br />
Me<br />
OMe<br />
instable<br />
BrMg<br />
CO + HCl<br />
Historically Formyl groups were added with the help of Orthoesters:<br />
Et Et<br />
O<br />
R MgX<br />
Et O in the presence of :<br />
Heat > 35°C<br />
and evaporate<br />
R MgX<br />
Et<br />
OEt<br />
HC OEt<br />
OEt<br />
Orthoester<br />
the ether<br />
OEt<br />
HC OEt<br />
OEt<br />
Stetter chose to change the orthoester in making<br />
one of the OEt group to a better leaving group a<br />
Phenol (OPh). This facilitates the reaction and it can<br />
be done now at r.t.<br />
OEt<br />
HC OEt<br />
OEt<br />
+ PhOH HC<br />
OEt<br />
OEt<br />
OPh<br />
+ HOEt<br />
Utilisation of mixed othoformiates: Chem. Ber. 1970, 103, 643<br />
Synthesis of Stetter<br />
MgBr<br />
OEt<br />
HC OEt<br />
OPh<br />
O<br />
<strong>Jasmone</strong><br />
R<br />
O<br />
H<br />
H<br />
H 3O<br />
O<br />
R<br />
H<br />
OEt<br />
OEt<br />
Highly reactive,<br />
will react immediately<br />
with the grignard<br />
reagent.<br />
R<br />
R MgX<br />
OEt<br />
H<br />
OEt<br />
OEt O<br />
H3O OEt<br />
NaOH<br />
10 mol%<br />
Thiazolium-Salt<br />
O<br />
4<br />
3<br />
O<br />
2<br />
1<br />
O