Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
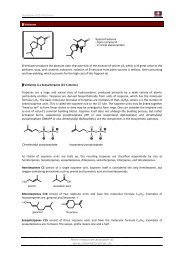
Synthesis of JASMONE<br />
Synthesis:<br />
EtO<br />
O<br />
O<br />
O<br />
O<br />
O<br />
1<br />
2<br />
H 2N NMe 2<br />
O<br />
100%<br />
90%<br />
+<br />
N NMe 2<br />
O<br />
10%<br />
Enolate formed at Position: 2 1<br />
electron poor due<br />
to conjugation<br />
R<br />
R<br />
O O<br />
E 2<br />
O<br />
E 1<br />
:<br />
1. O 3<br />
2. Me 2S<br />
O<br />
O<br />
Ph 3P<br />
Sterically hindered Base<br />
(Kinetically controlled deprotonation)<br />
NaOH<br />
to create the<br />
Ylide<br />
: Thermodynamically controlled deprotonation<br />
R<br />
R<br />
For E 1 = Br<br />
For E 2 =<br />
Br<br />
BH 4<br />
H 3O<br />
- CO 2<br />
O<br />
EtO<br />
EtO<br />
O O<br />
O O<br />
E 2<br />
R<br />
E 1<br />
R<br />
n-Bu Li<br />
More resources available at<br />
www.chemistforchrist.de<br />
Li<br />
E 2<br />
1. n-BuLi<br />
2.<br />
O<br />
3. H2O + Cu(OAc) 2<br />
O<br />
75%<br />
SiMe3 N<br />
SiMe3 EtO<br />
EtO<br />
E 1<br />
O O<br />
O O<br />
NaOH<br />
70%<br />
44% yield<br />
LMDS<br />
R<br />
O<br />
OH<br />
O<br />
O<br />
What was deprotonated<br />
last will react first!<br />
E 1<br />
R<br />
Oxidation<br />
with PCC