Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
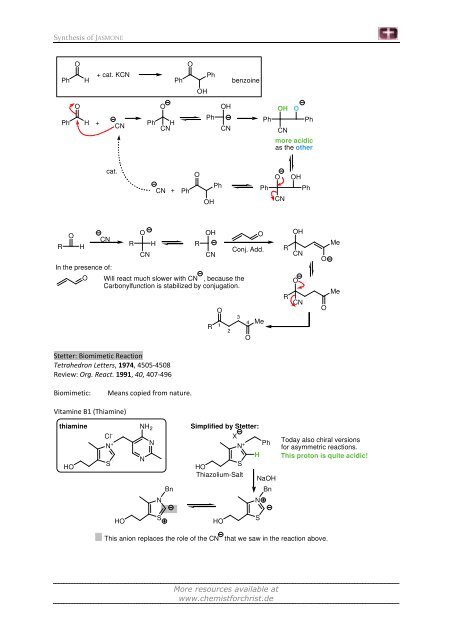
Synthesis of JASMONE<br />
R<br />
O<br />
Ph H<br />
Ph H<br />
O<br />
O<br />
H<br />
+ cat. KCN<br />
+<br />
CN<br />
In the presence of:<br />
cat.<br />
CN<br />
R<br />
O<br />
CN<br />
O<br />
Ph H<br />
CN<br />
H<br />
CN<br />
Ph<br />
+ Ph<br />
O<br />
O<br />
R<br />
OH<br />
Ph<br />
Ph<br />
OH<br />
OH<br />
CN<br />
Ph<br />
OH<br />
CN<br />
O Will react much slower with CN , because the<br />
Carbonylfunction is stabilized by conjugation.<br />
Stetter: Biomimetic Reaction<br />
Tetrahedron Letters, 1974, 4505-4508<br />
Review: Org. React. 1991, 40, 407-496<br />
Biomimetic: Means copied from nature.<br />
Vitamine B1 (Thiamine)<br />
thiamine<br />
HO<br />
Cl -<br />
N +<br />
S<br />
HO<br />
NH 2<br />
N<br />
N<br />
N<br />
S<br />
Bn<br />
O<br />
R 1<br />
benzoine<br />
Ph<br />
Ph<br />
O<br />
Conj. Add.<br />
More resources available at<br />
www.chemistforchrist.de<br />
2<br />
3<br />
4 Me<br />
O<br />
Simplified by Stetter:<br />
HO<br />
S<br />
Thiazolium-Salt<br />
HO<br />
X<br />
N +<br />
H<br />
Ph<br />
NaOH<br />
N<br />
S<br />
Bn<br />
OH<br />
CN<br />
O<br />
Ph<br />
more acidic<br />
as the other<br />
O<br />
CN<br />
R<br />
R<br />
OH<br />
OH<br />
CN<br />
O<br />
CN<br />
Ph<br />
O<br />
O<br />
Me<br />
Me<br />
Today also chiral versions<br />
for asymmetric reactions.<br />
This proton is quite acidic!<br />
This anion replaces the role of the CN that we saw in the reaction above.