Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Jasmone 1 - ChemistforChrist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
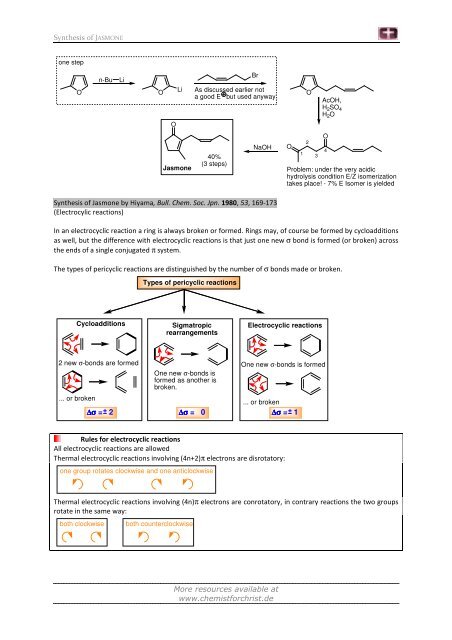
Synthesis of JASMONE<br />
one step<br />
O<br />
n-Bu Li<br />
O<br />
O<br />
Li<br />
<strong>Jasmone</strong><br />
Br<br />
As discussed earlier not<br />
a good E but used anyway<br />
40%<br />
(3 steps)<br />
NaOH<br />
Synthesis of <strong>Jasmone</strong> by Hiyama, Bull. Chem. Soc. Jpn. 1980, 53, 169-173<br />
(Electrocylic reactions)<br />
More resources available at<br />
www.chemistforchrist.de<br />
O<br />
1<br />
O<br />
2<br />
3<br />
AcOH,<br />
H 2SO 4<br />
H 2O<br />
O<br />
4<br />
Problem: under the very acidic<br />
hydrolysis condition E/Z isomerization<br />
takes place! - 7% E Isomer is yielded<br />
In an electrocyclic reaction a ring is always broken or formed. Rings may, of course be formed by cycloadditions<br />
as well, but the difference with electrocyclic reactions is that just one new σ bond is formed (or broken) across<br />
the ends of a single conjugated π system.<br />
The types of pericyclic reactions are distinguished by the number of σ bonds made or broken.<br />
Cycloadditions<br />
2 new σ-bonds are formed<br />
... or broken<br />
∆σ = 2<br />
Types of pericyclic reactions<br />
Sigmatropic<br />
rearrangements<br />
One new σ-bonds is<br />
formed as another is<br />
broken.<br />
∆σ = 0<br />
Electrocyclic reactions<br />
One new σ-bonds is formed<br />
... or broken<br />
∆σ = 1<br />
Rules for electrocyclic reactions<br />
All electrocyclic reactions are allowed<br />
Thermal electrocyclic reactions involving (4n+2)π electrons are disrotatory:<br />
one group rotates clockwise and one anticlockwise<br />
Thermal electrocyclic reactions involving (4n)π electrons are conrotatory, in contrary reactions the two groups<br />
rotate in the same way:<br />
both clockwise both counterclockwise