Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
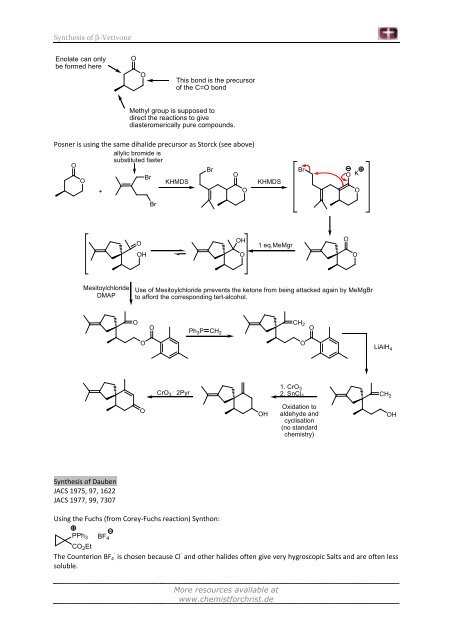
Enolate can only<br />
be formed here<br />
O<br />
O<br />
This bond is the precursor<br />
<strong>of</strong> the C=O bond<br />
Methyl group is supposed to<br />
direct the reactions to give<br />
diasteromerically pure compounds.<br />
Posner is using the same dihalide precursor as Storck (see above)<br />
allylic bromide is<br />
O<br />
O<br />
substituted faster<br />
Br<br />
KHMDS<br />
Br<br />
O<br />
KHMDS<br />
+<br />
O<br />
Mesitoylchloride<br />
DMAP<br />
<strong>Synthesis</strong> <strong>of</strong> Dauben<br />
JACS 1975, 97, 1622<br />
JACS 1977, 99, 7307<br />
O<br />
O<br />
OH<br />
O<br />
Br<br />
OH<br />
1 eq.MeMgr<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
Use <strong>of</strong> Mesitoylchloride prrevents the ketone from being attacked again by MeMgBr<br />
to afford the corresponding tert-alcohol.<br />
O<br />
CrO 3 . 2Pyr<br />
Ph 3P CH 2<br />
Using the Fuchs (from Corey-Fuchs reaction) Synthon:<br />
O<br />
OH<br />
Br<br />
CH 2 O<br />
O<br />
1. CrO 3<br />
2. SnCl 4<br />
Oxidation to<br />
aldehyde and<br />
cyclisation<br />
(no standard<br />
chemistry)<br />
O<br />
O<br />
K<br />
O<br />
O<br />
LiAlH 4<br />
PPh3 BF4<br />
CO2Et - -<br />
The Counterion BF4 is chosen because Cl and other halides <strong>of</strong>ten give very hygroscopic Salts and are <strong>of</strong>ten less<br />
soluble.<br />
CH 2<br />
OH