Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Synthesis of β-Vetivone More resources available ... - ChemistforChrist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Synthesis</strong> <strong>of</strong> <strong>β</strong>-<strong>Vetivone</strong><br />
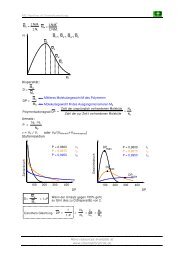
<strong>Synthesis</strong> <strong>of</strong> starting material:<br />
O<br />
OEt<br />
OEt<br />
Cl<br />
Cl<br />
+<br />
O<br />
OH<br />
OH<br />
OEt<br />
OEt<br />
O<br />
O<br />
OEt<br />
OEt<br />
O<br />
O<br />
The last step didn't work due to following side reaction:<br />
The fast ring closure is due to the a very reactive LG (Allylic chloride)<br />
OH<br />
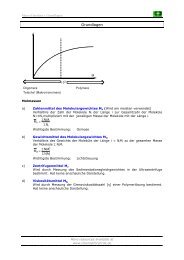
New approach:<br />
OH SO 2Cl<br />
OH 2 eq. n-BuLi<br />
OH<br />
O<br />
Cl<br />
OH<br />
LiAlH 4<br />
O 2 eq. MsCl<br />
O<br />
<strong>More</strong> <strong>resources</strong> <strong>available</strong> at<br />
www.chemistforchrist.de<br />
O<br />
+<br />
O<br />
OH<br />
OH<br />
Reacts faster due to being<br />
the less stabilized alcoholate<br />
Yields are rather moderate but the dichloride could be obtained.<br />
Revisiting dipolar, aprotic solvents<br />
Me<br />
O<br />
S<br />
Me<br />
H<br />
O<br />
N Me<br />
Me<br />
DMSO DMF<br />
Most important ones are DMSO and DMF<br />
Cl<br />
Cl<br />
SO 2Cl<br />
O<br />
OMs<br />
HMPA<br />
LiCl<br />
Cl<br />
Cl<br />
The allylic alcoholate<br />
is not very reactive and<br />
give is therefore quickly<br />
Mesylated before<br />
undergoing ring -<br />
closure.<br />
OMs<br />
OMs<br />
HMPA makes the Cl -<br />
very nucleophilic.