Hetron-Ashland Resins
Hetron-Ashland Resins
Hetron-Ashland Resins
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
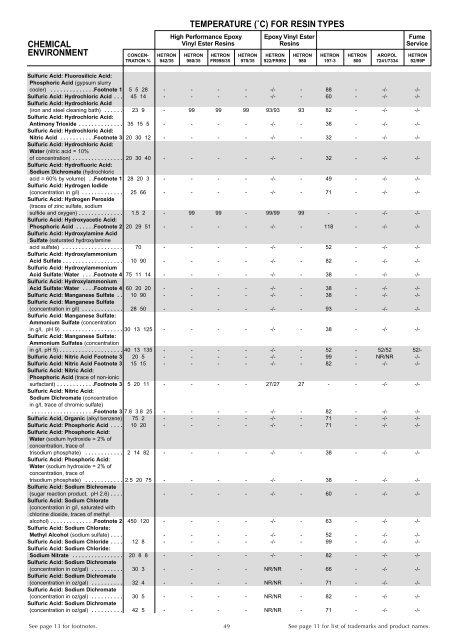
CHEMICAL<br />
ENVIRONMENT<br />
TEMPERATURE (˚C) FOR RESIN TYPES<br />
High Performance Epoxy Epoxy Vinyl Ester Fume<br />
Vinyl Ester <strong>Resins</strong> <strong>Resins</strong> Service<br />
CONCEN- HETRON HETRON HETRON HETRON HETRON HETRON HETRON HETRON AROPOL HETRON<br />
TRATION % 942/35 980/35 FR998/35 970/35 922/FR992 980 197-3 800 7241/7334 92/99P<br />
Sulfuric Acid: Fluorosilicic Acid:<br />
Phosphoric Acid (gypsum slurry<br />
cooler) . . . . . . . . . . . . . .Footnote 1 5 5 28 - - - - -/- - 88 - -/- -/-<br />
Sulfuric Acid: Hydrochloric Acid . . . 45 14 - - - - -/- - 60 - -/- -/-<br />
Sulfuric Acid: Hydrochloric Acid<br />
(iron and steel cleaning bath) . . . . . . 23 9 - 99 99 99 93/93 93 82 - -/- -/-<br />
Sulfuric Acid: Hydrochloric Acid:<br />
Antimony Trioxide . . . . . . . . . . . . . . 35 15 5 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Hydrochloric Acid:<br />
Nitric Acid . . . . . . . . . . .Footnote 3 20 30 12 - - - - -/- - 32 - -/- -/-<br />
Sulfuric Acid: Hydrochloric Acid:<br />
Water (nitric acid = 10%<br />
of concentration) . . . . . . . . . . . . . . . . 20 30 40 - - - - -/- - 32 - -/- -/-<br />
Sulfuric Acid: Hydrofluoric Acid:<br />
Sodium Dichromate (hydrochloric<br />
acid = 60% by volume) . .Footnote 1 28 20 3 - - - - -/- - 49 - -/- -/-<br />
Sulfuric Acid: Hydrogen Iodide<br />
(concentration in g/l) . . . . . . . . . . . . . 25 66 - - - - -/- - 71 - -/- -/-<br />
Sulfuric Acid: Hydrogen Peroxide<br />
(traces of zinc sulfate, sodium<br />
sulfide and oxygen) . . . . . . . . . . . . . . 1.5 2 - 99 99 - 99/99 99 - - -/- -/-<br />
Sulfuric Acid: Hydroxyacetic Acid:<br />
Phosphoric Acid . . . . . .Footnote 2 20 29 51 - - - - -/- - 118 - -/- -/-<br />
Sulfuric Acid: Hydroxylamine Acid<br />
Sulfate (saturated hydroxylamine<br />
acid sulfate) . . . . . . . . . . . . . . . . . . . 70 - - - - -/- - 52 - -/- -/-<br />
Sulfuric Acid: Hydroxylammonium<br />
Acid Sulfate . . . . . . . . . . . . . . . . . . . 10 90 - - - - -/- - 82 - -/- -/-<br />
Sulfuric Acid: Hydroxylammonium<br />
Acid Sulfate: Water . . . .Footnote 4 75 11 14 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Hydroxylammonium<br />
Acid Sulfate: Water . . . .Footnote 4 60 20 20 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Manganese Sulfate . . 10 90 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Manganese Sulfate<br />
(concentration in g/l) . . . . . . . . . . . . . 28 50 - - - - -/- - 93 - -/- -/-<br />
Sulfuric Acid: Manganese Sulfate:<br />
Ammonium Sulfate (concentration<br />
in g/l, pH 9) . . . . . . . . . . . . . . . . . . . 30 13 125 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Manganese Sulfate:<br />
Ammonium Sulfates (concentration<br />
in g/l, pH 5) . . . . . . . . . . . . . . . . . . . . 40 13 135 - - - - -/- - 52 - 52/52 52/-<br />
Sulfuric Acid: Nitric Acid Footnote 3 20 5 - - - - -/- - 99 - NR/NR -/-<br />
Sulfuric Acid: Nitric Acid Footnote 3 15 15 - - - - -/- - 82 - -/- -/-<br />
Sulfuric Acid: Nitric Acid:<br />
Phosphoric Acid (trace of non-ionic<br />
surfactant) . . . . . . . . . . . .Footnote 3 5 20 11 - - - - 27/27 27 - - -/- -/-<br />
Sulfuric Acid: Nitric Acid:<br />
Sodium Dichromate (concentration<br />
in g/l, trace of chromic sulfate)<br />
. . . . . . . . . . . . . . . . . . . .Footnote 3 7.8 3.8 25 - - - - -/- - 82 - -/- -/-<br />
Sulfuric Acid, Organic (alkyl benzene) 75 2 - - - - -/- - 71 - -/- -/-<br />
Sulfuric Acid: Phosphoric Acid . . . . 10 20 - - - - -/- - 71 - -/- -/-<br />
Sulfuric Acid: Phosphoric Acid:<br />
Water (sodium hydroxide = 2% of<br />
concentration, trace of<br />
trisodium phosphate) . . . . . . . . . . . . 2 14 82 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Phosphoric Acid:<br />
Water (sodium hydroxide = 2% of<br />
concentration, trace of<br />
trisodium phosphate) . . . . . . . . . . . . 2.5 20 75 - - - - -/- - 38 - -/- -/-<br />
Sulfuric Acid: Sodium Bichromate<br />
(sugar reaction product, pH 2.6) . . . . - - - - -/- - 60 - -/- -/-<br />
Sulfuric Acid: Sodium Chlorate<br />
(concentration in g/l, saturated with<br />
chlorine dioxide, traces of methyl<br />
alcohol) . . . . . . . . . . . . . .Footnote 2 450 120 - - - - -/- - 63 - -/- -/-<br />
Sulfuric Acid: Sodium Chlorate:<br />
Methyl Alcohol (sodium sulfate) . . . . - - - - -/- - 52 - -/- -/-<br />
Sulfuric Acid: Sodium Chloride . . . . 12 8 - - - - -/- - 99 - -/- -/-<br />
Sulfuric Acid: Sodium Chloride:<br />
Sodium Nitrate . . . . . . . . . . . . . . . . 20 8 8 - - - - -/- - 82 - -/- -/-<br />
Sulfuric Acid: Sodium Dichromate<br />
(concentration in oz/gal) . . . . . . . . . . 30 3 - - - - NR/NR - 66 - -/- -/-<br />
Sulfuric Acid: Sodium Dichromate<br />
(concentration in oz/gal) . . . . . . . . . . 32 4 - - - - NR/NR - 71 - -/- -/-<br />
Sulfuric Acid: Sodium Dichromate<br />
(concentration in oz/gal) . . . . . . . . . . 30 5 - - - - NR/NR - 82 - -/- -/-<br />
Sulfuric Acid: Sodium Dichromate<br />
(concentration in oz/gal) . . . . . . . . . . 42 5 - - - - NR/NR - 71 - -/- -/-<br />
See page 13 for footnotes. 49<br />
See page 11 for list of trademarks and product names.











![[ES] - LUPEROX K10 - 2008-04-02](https://img.yumpu.com/40905446/1/184x260/es-luperox-k10-2008-04-02.jpg?quality=85)




