Sterols and the phytosterol content in oilseed rape - Journal of Cell ...
Sterols and the phytosterol content in oilseed rape - Journal of Cell ...
Sterols and the phytosterol content in oilseed rape - Journal of Cell ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Animal cells conta<strong>in</strong> only one major sterol, i.e.<br />
c h o l e s t e ro l (Figure 2a). Cholesterol also occurs,<br />
though only <strong>in</strong> a few percentage <strong>of</strong> <strong>the</strong> whole sterol<br />
<strong>content</strong>, <strong>in</strong> plants (Gordon <strong>and</strong> Miller, 1997).<br />
Chemically, it is an analogue to <strong>the</strong> <strong>phytosterol</strong>s,<br />
differ<strong>in</strong>g only <strong>in</strong> <strong>the</strong> side cha<strong>in</strong>. Fungal cells, toge<strong>the</strong>r<br />
with some unicellular algae <strong>and</strong> lichens syn<strong>the</strong>sise<br />
ergosterol - provitam<strong>in</strong> D2 (Grunwald, 1980; Rehm<br />
<strong>and</strong> Espig, 1991). Namely, ergocalciferol (vitam<strong>in</strong> D2)<br />
is produced by ultraviolet irradiation <strong>of</strong> provitam<strong>in</strong> D2<br />
( e rgosterol), which occurs <strong>in</strong> yeast <strong>and</strong> fungi (Figure 2b).<br />
In contrast to animal cells <strong>and</strong> fungi, plant cells<br />
syn<strong>the</strong>size complex array <strong>of</strong> <strong>phytosterol</strong> mixtures with<br />
<strong>the</strong> sterol pr<strong>of</strong>iles vary<strong>in</strong>g between species.<br />
The scientific names <strong>of</strong> <strong>phytosterol</strong>s are given to<br />
<strong>the</strong>m accord<strong>in</strong>g to <strong>the</strong> number <strong>of</strong> C atoms <strong>in</strong> <strong>the</strong> C-17<br />
side cha<strong>in</strong>, <strong>the</strong> number <strong>and</strong> <strong>the</strong> position <strong>of</strong> <strong>the</strong> double<br />
<strong>Sterols</strong> <strong>and</strong> <strong>phytosterol</strong>s <strong>in</strong> <strong>oilseed</strong> <strong>rape</strong> 73<br />
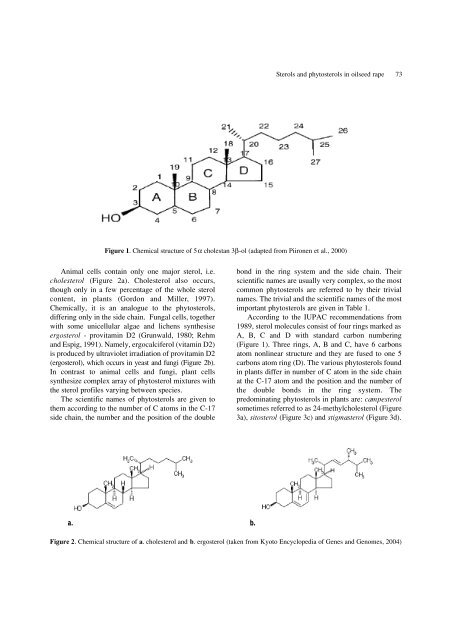
Figure 1. Chemical structure <strong>of</strong> 5 α cholestan 3β-ol (adapted from Piironen et al., 2000)<br />
a. b.<br />
bond <strong>in</strong> <strong>the</strong> r<strong>in</strong>g system <strong>and</strong> <strong>the</strong> side cha<strong>in</strong>. Their<br />
scientific names are usually very complex, so <strong>the</strong> most<br />
common <strong>phytosterol</strong>s are referred to by <strong>the</strong>ir trivial<br />
names. The trivial <strong>and</strong> <strong>the</strong> scientific names <strong>of</strong> <strong>the</strong> most<br />
important <strong>phytosterol</strong>s are given <strong>in</strong> Table 1.<br />
Accord<strong>in</strong>g to <strong>the</strong> IUPAC recommendations from<br />
1989, sterol molecules consist <strong>of</strong> four r<strong>in</strong>gs marked as<br />
A, B, C <strong>and</strong> D with st<strong>and</strong>ard carbon number<strong>in</strong>g<br />
(Figure 1). Three r<strong>in</strong>gs, A, B <strong>and</strong> C, have 6 carbons<br />
atom nonl<strong>in</strong>ear structure <strong>and</strong> <strong>the</strong>y are fused to one 5<br />
carbons atom r<strong>in</strong>g (D). The various <strong>phytosterol</strong>s found<br />
<strong>in</strong> plants differ <strong>in</strong> number <strong>of</strong> C atom <strong>in</strong> <strong>the</strong> side cha<strong>in</strong><br />
at <strong>the</strong> C-17 atom <strong>and</strong> <strong>the</strong> position <strong>and</strong> <strong>the</strong> number <strong>of</strong><br />
<strong>the</strong> double bonds <strong>in</strong> <strong>the</strong> r<strong>in</strong>g system. T h e<br />
predom<strong>in</strong>at<strong>in</strong>g <strong>phytosterol</strong>s <strong>in</strong> plants are: campesterol<br />
sometimes referred to as 24-methylcholesterol (Figure<br />
3a), sitosterol (Figure 3c) <strong>and</strong> stigmasterol (Figure 3d).<br />
Figure 2. Chemical structure <strong>of</strong> a. cholesterol <strong>and</strong> b. ergosterol (taken from Kyoto Encyclopedia <strong>of</strong> Genes <strong>and</strong> Genomes, 2004)