a thermodynamic approach to cement hydration - Eawag-Empa ...
a thermodynamic approach to cement hydration - Eawag-Empa ...
a thermodynamic approach to cement hydration - Eawag-Empa ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
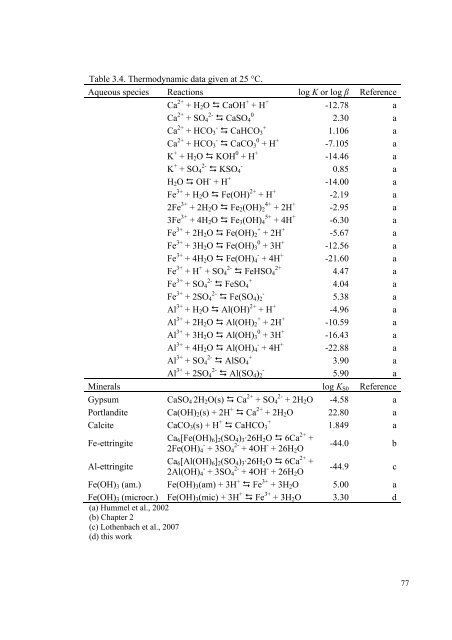
Table 3.4. Thermodynamic data given at 25 °C.<br />
Aqueous species Reactions log K or log β Reference<br />
Ca 2+ + H2O CaOH + + H +<br />
-12.78 a<br />
Ca 2+ + SO4 2- CaSO4 0<br />
2.30 a<br />
Ca 2+ + HCO3 - CaHCO3 +<br />
1.106 a<br />
Ca 2+ + HCO3 - CaCO3 0 + H +<br />
-7.105 a<br />
K + + H2O KOH 0 + H +<br />
-14.46 a<br />
K + + SO4 2- KSO4 -<br />
0.85 a<br />
H2O OH - + H +<br />
-14.00 a<br />
Fe 3+ + H2O Fe(OH) 2+ + H +<br />
-2.19 a<br />
2Fe 3+ + 2H2O Fe2(OH)2 4+ + 2H +<br />
-2.95 a<br />
3Fe 3+ + 4H2O Fe3(OH)4 5+ + 4H +<br />
-6.30 a<br />
Fe 3+ + 2H2O Fe(OH)2 + + 2H +<br />
-5.67 a<br />
Fe 3+ + 3H2O Fe(OH)3 0 + 3H +<br />
-12.56 a<br />
Fe 3+ + 4H2O Fe(OH)4 - + 4H +<br />
-21.60 a<br />
Fe 3+ + H + + SO4 2- FeHSO4 2+<br />
4.47 a<br />
Fe 3+ + SO4 2- FeSO4 +<br />
4.04 a<br />
Fe 3+ + 2SO4 2- Fe(SO4)2 -<br />
5.38 a<br />
Al 3+ + H2O Al(OH) 2+ + H +<br />
-4.96 a<br />
Al 3+ + 2H2O Al(OH)2 + + 2H +<br />
-10.59 a<br />
Al 3+ + 3H2O Al(OH)3 0 + 3H +<br />
-16.43 a<br />
Al 3+ + 4H2O Al(OH)4 - + 4H +<br />
-22.88 a<br />
Al 3+ + SO4 2- AlSO4 +<br />
3.90 a<br />
Al 3+ + 2SO4 2- Al(SO4)2 -<br />
5.90 a<br />
Minerals log KS0 Reference<br />
Gypsum CaSO4 . 2H2O(s) Ca 2+ + SO4 2- + 2H2O -4.58 a<br />
Portlandite Ca(OH)2(s) + 2H + Ca 2+ + 2H2O 22.80 a<br />
Calcite CaCO3(s) + H + CaHCO3 +<br />
1.849 a<br />
Fe-ettringite<br />
Ca6[Fe(OH)6]2(SO4)3·26H2O 6Ca 2+ +<br />
2Fe(OH)4 - + 3SO4 2- + 4OH - + 26H2O<br />
-44.0 b<br />
Al-ettringite<br />
Ca6[Al(OH)6]2(SO4)3·26H2O 6Ca 2+ +<br />
2Al(OH)4 - + 3SO4 2- + 4OH - + 26H2O<br />
-44.9 c<br />
Fe(OH)3 (am.) Fe(OH)3(am) + 3H + Fe 3+ + 3H2O 5.00 a<br />
Fe(OH)3 (microcr.) Fe(OH)3(mic) + 3H + Fe 3+ + 3H2O<br />
(a) Hummel et al., 2002<br />
(b) Chapter 2<br />
(c) Lothenbach et al., 2007<br />
(d) this work<br />
3.30 d<br />
77