Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
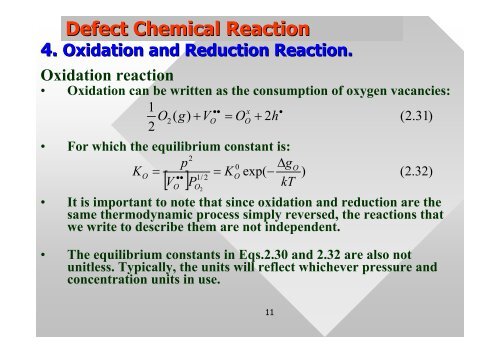
Defect Chemical Reaction<br />
4. Oxidation and Reduction Reaction.<br />
Oxidation reaction<br />
• Oxidation can be written as the consumption of oxygen vacancies:<br />
1 2<br />
O<br />
2<br />
••<br />
x<br />
( g)<br />
+ V = O + 2h<br />
• For which the equilibrium constant is:<br />
2<br />
p<br />
0 ∆g<br />
KO<br />
= = K exp(<br />
1/<br />
2 O −<br />
••<br />
V P<br />
kT<br />
[ ]<br />
O<br />
O<br />
2<br />
O<br />
• It is important to note that s<strong>in</strong>ce oxidation and reduction are the<br />
same thermodynamic process simply reversed, the reactions that<br />
we write to describe them are not <strong>in</strong>dependent.<br />
• The equilibrium constants <strong>in</strong> Eqs.2.30 and 2.32 are also not<br />
unitless. Typically, the units will reflect whichever pressure and<br />
concentration units <strong>in</strong> use.<br />
O<br />
11<br />
•<br />
O<br />
)<br />
( 2.<br />
31)<br />
( 2.<br />
32)