Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
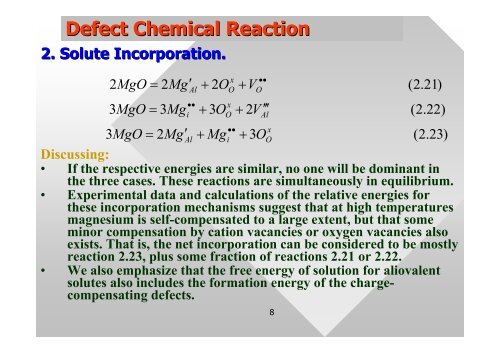
Defect Chemical Reaction<br />
2. Solute Incorporation.<br />
x •<br />
2MgO<br />
= 2Mg′<br />
Al + 2OO<br />
+ VO<br />
••<br />
x<br />
3MgO = 3Mgi<br />
+ 3OO<br />
+ 2V<br />
′<br />
Al<br />
3MgO<br />
= 2Mg′<br />
Al<br />
Discuss<strong>in</strong>g:<br />
••<br />
x<br />
+ Mgi<br />
+ 3OO<br />
( 2.<br />
23)<br />
• If the respective energies are similar, no one will be dom<strong>in</strong>ant <strong>in</strong><br />
the three cases. These reactions are simultaneously <strong>in</strong> equilibrium.<br />
• Experimental data and calculations of the relative energies for<br />
these <strong>in</strong>corporation mechanisms suggest that at high temperatures<br />
magnesium is self-compensated to a large extent, but that some<br />
m<strong>in</strong>or compensation by cation vacancies or oxygen vacancies also<br />
exists. That is, the net <strong>in</strong>corporation can be considered to be mostly<br />
reaction 2.23, plus some fraction of reactions 2.21 or 2.22.<br />
• We also emphasize that the free energy of solution for aliovalent<br />
solutes also <strong>in</strong>cludes the formation energy of the chargecompensat<strong>in</strong>g<br />
<strong>defect</strong>s.<br />
•<br />
8<br />
( 2.<br />
21)<br />
( 2.<br />
22)