Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Introduction into defect studies in ceramic materials(III) - Positron ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
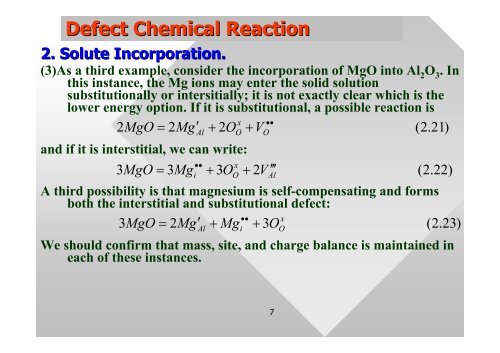
Defect Chemical Reaction<br />
2. Solute Incorporation.<br />
(3)As a third example, consider the <strong>in</strong>corporation of MgO <strong><strong>in</strong>to</strong> Al2O3 . In<br />
this <strong>in</strong>stance, the Mg ions may enter the solid solution<br />
substitutionally or <strong>in</strong>tersitially; it is not exactly clear which is the<br />
lower energy option. If it is substitutional, a possible reaction is<br />
x ••<br />
2MgO<br />
= 2Mg′<br />
Al + 2OO<br />
+ VO<br />
( 2.<br />
21)<br />
and if it is <strong>in</strong>terstitial, we can write:<br />
••<br />
x<br />
3MgO = 3Mgi<br />
+ 3OO<br />
+ 2V<br />
′<br />
Al<br />
( 2.<br />
22)<br />
A third possibility is that magnesium is self-compensat<strong>in</strong>g and forms<br />
both the <strong>in</strong>terstitial and substitutional <strong>defect</strong>:<br />
3MgO<br />
= 2Mg′<br />
••<br />
x<br />
+ Mg + 3O<br />
( 2.<br />
23)<br />
Al<br />
i<br />
We should confirm that mass, site, and charge balance is ma<strong>in</strong>ta<strong>in</strong>ed <strong>in</strong><br />
each of these <strong>in</strong>stances.<br />
7<br />
O