ReOss® Powder - Intra-Lock
ReOss® Powder - Intra-Lock
ReOss® Powder - Intra-Lock
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ReOss ® <strong>Powder</strong><br />
Indication<br />
ReOss ® is indicated for use in filling and/or augmenting intraoral/maxillofacial osseous defects; such as infrabony/intrabony<br />
periodontal osseous defects, furcation defects, alveolar ridge osseous defects, tooth extraction sites and in sinus elevation<br />
procedures.<br />
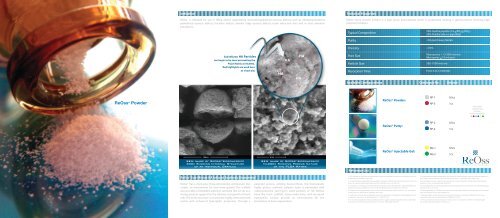
Sub-Micron HA Particles<br />
can begin to be seen permeating the<br />
PGLA Matrix at 50,000x.<br />
Red highlights are used here<br />
as visual aid.<br />
SEM Image of ReOss ® Biocomposite<br />
300x Showing Internal Structure<br />
of an Individual Granule<br />
<strong>ReOss®</strong> Sub-Micron HA Infused Synthetic Biomaterial<br />
<strong>ReOss®</strong> has a multi-pore three-dimensional architecture that<br />
creates an environment for new bone growth. The scaffold<br />
also provides a hospitable adhesive substrate that serves as a<br />
strong physical support for the infusion and growth of bone<br />
cells. The entire structure is an intricate, highly interconnected<br />
matrix with enhanced hydrophilic properties. Through a<br />
HA<br />
HA<br />
HA<br />
HA<br />
SEM Image of ReOss ® Biocomposite<br />
10,000x Showing Porous Nature<br />
of the PLGA Matrix<br />
patented process utilizing barosynthesis, the biomaterial’s<br />
highly porous, synthetic polymer foam is permeated with<br />
osteoconductive sub-micron sized particles of HA. ReOss’s<br />
bone-like foam scaffold, osteoconductivity, and increased<br />
hydrophilic surface provide an environment for the<br />
stimulation of bone regeneration.<br />
Technical Properties<br />
ReOss<br />
<strong>ReOss®</strong><br />
Bone<br />
Bone<br />
Growth<br />
Growth<br />
Initiator<br />
Initiator<br />
is a<br />
is<br />
high<br />
a high<br />
purity<br />
purity<br />
biocomposite<br />
biocomposite<br />
which<br />
which<br />
is synthesized<br />
is synthesized<br />
utilizing<br />
utilizing<br />
a special<br />
a special<br />
process<br />
process<br />
involving<br />
involving<br />
high<br />
high<br />
pressure pressure formation. formation.<br />
Typical Composition<br />
Purity<br />
Porosity<br />
Pore Size<br />
Particle Size Size<br />
Resorption Time Time<br />
50% Hydroxyapatite (Ca ( Ca10(PO4)6 10(PO4) 6(OH) (OH)2) 2)<br />
50% Poly(lactide-co-glycolide)<br />
< 70 %<br />
Macropores = 15-300 150- 300 microns<br />
Micropores < ≤10 10 microns<br />
300-750 500 -1000 Microns microns<br />
From 6 to 12 Months<br />
Available Configurations Catalogue No. No. Volume<br />
Reference List<br />
<strong>ReOss®</strong> ReOss<strong>Powder</strong>: <strong>Powder</strong>:<br />
<strong>ReOss®</strong> ReOssPutty: Putty:<br />
ReOss <strong>ReOss®</strong> Injectable Gel:<br />
1. 1. Poly Poly (lactide-co-glycolide)/hydroxyapatite (lactide-co-glycolide)/hydroxyapatite composite composite scaffolds for bone tissue tissue engineering. engineering. Kim Kimet et<br />
al.<br />
al.<br />
Biomaterials<br />
Biomaterials<br />
27(2006)<br />
27(2006)<br />
1399-1409.<br />
1399-1409.<br />
2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity.<br />
2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity.<br />
Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215.<br />
Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215.<br />
3. Comparison of Osteogenic Potential Between Apatite-Coated Poly (lactide-co-glycolide)/Hydroxy<br />
apatite 3. Comparison Particulates of Osteogenic and Bio-Oss. Potential Kim et Between al. Dental Apatite-Coated Materials Journal Poly 2008; (lactide-co-glycolide)/Hydroxy-<br />
27(3): 368-375.<br />
4.<br />
apatite<br />
Peripheral<br />
Particulates<br />
nerve regeneration<br />
and Bio-Oss.<br />
within<br />
Kim et al.<br />
an<br />
Dental<br />
asymmetrically<br />
Materials<br />
porous<br />
Journal<br />
PLGA/<br />
2008;<br />
Pluronic<br />
27(3): 368-375.<br />
F127 nerve guide<br />
conduit. 4. Peripheral Oh et nerve al. Biomaterials regeneration 29(2008) within 1601-1609<br />
an asymmetrically porous PLGA/ Pluronic F127 nerve guide<br />
5. conduit. Sterilization, Oh ettoxicity, al. Biomaterials biocompatibility 29(2008) 1601-1609 and clinical applications of polylactic acid/polyglycolic acid<br />
copolymers.<br />
5. Sterilization,<br />
Athanasiou<br />
toxicity, biocompatibility<br />
et al. Biomaterials<br />
and<br />
17<br />
clinical<br />
(1996)<br />
applications<br />
93-102<br />
of polylactic acid/polyglycolic acid<br />
6. copolymers. In vitro biocompatibility Athanasiou et of al. bioresorbable Biomaterials 17 polymers: (1996) 93-102 poly(L-lactide) and poly(lactide-co-glycolide).<br />
A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839<br />
6. In vitro biocompatibility of bioresorbable polymers: poly(L-lactide) and poly(lactide-co-glycolide).<br />
A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839<br />
RP-1 0.5cc 0.5 cc<br />
RP-3 RP-3<br />
1cc 1cc<br />
RP-2 0.5cc 0.5 cc<br />
RP-4 RP-4<br />
1cc1<br />
cc<br />
RG-1 0.5cc 0.5 cc<br />
RG-2 RG-2<br />
1cc1<br />
cc<br />
Color coded<br />
container caps<br />
for easier<br />
product recognition<br />
••••••<br />
SUB-MICRON HA INFUSED SYNTHETIC BIOMATERIAL<br />
7. 7. Biodegradation Biodegradation and and biocompatibility biocompatibility of of PLA PLA and and PLGA PLGA microspheres. James M. Anderson, Matthew Matthew S. S.<br />
Shiva<br />
Shiva<br />
Advanced<br />
Advanced<br />
Drug<br />
Drug<br />
Delivery<br />
Delivery<br />
Reviews<br />
Reviews<br />
Volume<br />
Volume<br />
28,<br />
28,<br />
Issue<br />
Issue<br />
1,<br />
1,<br />
13<br />
13<br />
October<br />
October<br />
1997,<br />
1997,<br />
Pages<br />
Pages<br />
5-24<br />
5-24<br />
8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials,<br />
8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials,<br />
Volume 25, Issue 18, Aug 2004, pp 3963-3972.<br />
Volume 25, Issue 18, Aug 2004, pp 3963-3972.<br />
9. The biodegradation of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology<br />
9. Volume The biodegradation 65, No 1, pp 43-48 of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology<br />
Volume<br />
10. Biocompatibility<br />
65, No 1, pp 43-48<br />
of Scaffold Components and Human Bone Fetal Cells. Montjovent et al. European<br />
10. Cells Biocompatibility and Material Vol.5. of Scaffold Suppl. Components 2,2003 p.79 and Human Bone Fetal Cells. Montjovent et al. European<br />
Cells 11. Biodegradable and Material Vol.5. and Suppl. bioactive 2,2003 porous p.79polymer/inorganic<br />
composite scaffolds for bone tissue<br />
11.<br />
engineering.<br />
Biodegradable<br />
Rezwan<br />
and<br />
et<br />
bioactive<br />
al. Biomaterials<br />
porous polymer/inorganic<br />
27(2006) 3413-3431<br />
composite scaffolds for bone tissue<br />
engineering. 12. Physico/Chemical Rezwan et characterization, al. Biomaterials 27(2006) in vitro and 3413-3431 in vivo evaluation of <strong>ReOss®</strong> and Synthograft<br />
particulate grafting materials. Coimbra et al.<br />
12. Physico/Chemical characterization, in vitro and in vivo evaluation of ReOss and Synthograft<br />
particulate grafting materials. Coimbra et al.
Barosynthesized BioComposite<br />
• 100% Synthetic<br />
• Osteo Adhesive Topography<br />
• Strong 3-D Scaffold<br />
• Osteoconductive<br />
• Enhanced Hydrophilicity<br />
<strong>ReOss®</strong> is hydrophilic and configured as a multi-pore three-dimensional scaffold engineered to integrate with the<br />
physiochemical state of bone tissue.<br />
Overview of <strong>ReOss®</strong>: A Resorbable Bone-like Biocomposite PLGA/HA:<br />
Poly (lactic-co-glycolic) acid / Hydroxyapatite<br />
<strong>ReOss®</strong> is a composite biomaterial comprised of two phases - a PLGA biodegradable polymer and a bioceramic. The polymer<br />
provides a structurally stable, porous and biocompatible 5,6,7 three-dimensional matrix to which biological fluids can penetrate,<br />
and cells can adhere.The HA bioceramic, due to its chemical and structural similarity to the mineral phase of native bone, allows<br />
the biocomposite to create a bond with the living host bone. 2,11<br />
Sub-Micron-HA Particles<br />
In order to improve the bioactivity of the ceramic phase, <strong>ReOss®</strong> utilizes Sub-Micron-sized particulate Hydroxyapatite (HA).<br />
This particulate size HA shows improved osteointegration and faster degradation times over larger particulate HA, which<br />
can impede bone growth because of its slow biodegradation. 1,2 Sub-Micron-sized HA also has been reported to augment<br />
protein adsorption and cell adhesion, further improving the ability for bone to regenerate. 2<br />
Multi-pore Resorbable Structure<br />
The porosity of the polymer matrix of <strong>ReOss®</strong> also provides an excellent environment to aid in stimulating bone regeneration.<br />
Through a patented process involving high-pressure formation of the polymer matrix, <strong>ReOss®</strong> is replete with both macro and<br />
micropores. The micropores allow biological fluids and small molecules, which aid in cell growth to perfuse the matrix,<br />
enveloping and sustaining the osteogenic cells that attach to the macropores of the scaffolds. As the cells begin to grow and<br />
develop, both phases of the biocomposite degrade, leaving behind a stable, natural bone matrix. 7,8,9<br />
Osteodynamics<br />
Several studies have shown that biodegradable polymer/bioceramic composites can improve bone regeneration as<br />
compared with conventional composites by optimizing controlled resorption, osteogenesis and osteointegration. 1,2,11 By<br />
controlling the parameters which determine the characteristics of resorption and osteoinductivity, <strong>ReOss®</strong> provides a superior<br />
vector for the stimulation of new bone growth.<br />
GLOBAL HEADQUARTERS<br />
6560 West Rogers Circle, Bldg. 24<br />
Boca Raton, Florida 33487 USA<br />
GLOBAL Tel: 877-330-0338<br />
HEADQUARTERS<br />
www.intra-lock.com • info@intra-lock.com<br />
6560 West Rogers Circle, Bldg. 24 • Boca Raton, Florida 33487 USA<br />
Tel: 877-330-0338<br />
www.intra-lock.com • info@intra-lock.com<br />
<strong>Intra</strong>-<strong>Lock</strong><br />
<strong>Intra</strong>-<strong>Lock</strong>® is registered trademark of <strong>Intra</strong>-<strong>Lock</strong>® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
® and ReOss ® are registered trademarks of <strong>Intra</strong>-<strong>Lock</strong> ® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
GLOBAL HEADQUARTERS<br />
6560 West Rogers Circle, Bldg. 24 • Boca Raton, Florida 33487 USA<br />
Tel: 877-330-0338<br />
www.intra-lock.com • info@intra-lock.com<br />
<strong>Intra</strong>-<strong>Lock</strong>® is registered trademark of <strong>Intra</strong>-<strong>Lock</strong>® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
CE 0499 0499 • EC • Rep: EC Rep: <strong>Intra</strong>-<strong>Lock</strong> System Europa, Spa., Srl., I-84100 Salerno • • © © Copyright 2008, 2009, <strong>Intra</strong>-<strong>Lock</strong>® <strong>Intra</strong>-<strong>Lock</strong> System International • S4EN-08-10CE<br />
0499 • EC Rep: <strong>Intra</strong>-<strong>Lock</strong> System Europa, Spa., I-84100 Salerno • © Copyright 2008, <strong>Intra</strong>-<strong>Lock</strong>® System International • S4EN-08-10<br />
® CE<br />
System International • S4EN-09-07<br />
SUB-MICRON HA INFUSED SYNTHETIC BIOMATERIAL