Problem Set #2 ? Induction in C. elegans embryos Zoology 470 ...
Problem Set #2 ? Induction in C. elegans embryos Zoology 470 ...
Problem Set #2 ? Induction in C. elegans embryos Zoology 470 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Problem</strong> <strong>Set</strong> <strong>#2</strong> – <strong>Induction</strong> <strong>in</strong> C. <strong>elegans</strong> <strong>embryos</strong><br />
<strong>Zoology</strong> <strong>470</strong> – Spr<strong>in</strong>g 2013<br />
20 Po<strong>in</strong>ts Total<br />
<strong>Problem</strong> <strong>Set</strong> Guidel<strong>in</strong>es<br />
1. Due date: This problem set is due by the end of class on Monday, April 1, 2013 (no fool<strong>in</strong>g!)<br />
2. Sources: You may use any sources at your disposal to answer the follow<strong>in</strong>g questions.<br />
Legitimate sources <strong>in</strong>clude classmates, knowledgeable friends and colleagues, written<br />
documents, and any other scientific resources you f<strong>in</strong>d useful. If you work with other<br />
classmates on this problem set, we ask that you list the other students with whom you<br />
worked to answer these questions. Although you may discuss these questions as part of a<br />
group, you are expected to answer the questions as an <strong>in</strong>dividual. If you believe that<br />
published references will help you answer these questions, you may cite those references.<br />
However, citation of additional references is not required, nor is it expected.<br />
3. Answer<strong>in</strong>g the questions: This problem set is designed to be answered concisely. Brief but<br />
complete answers should be written <strong>in</strong> the space provided. It is acceptable to type your<br />
answers, but you must provide a hard copy of the document you generate. Where you are asked<br />
for explanations, you must provide them to receive full credit. If you f<strong>in</strong>d it helpful, feel free to<br />
<strong>in</strong>clude diagrams <strong>in</strong> your answers. You need only turn <strong>in</strong> your answers on pages 2 & 3.<br />
Necessary <strong>in</strong>formation: All of the <strong>in</strong>formation and techniques needed to answer the questions on<br />
p. 2-3 have been presented <strong>in</strong> class, or are to be found <strong>in</strong> Gilbert's Developmental Biology. This<br />
problem set requires you to learn about a new signal<strong>in</strong>g pathway, the Notch/Delta pathway, which<br />
is described <strong>in</strong> Chapter 3 of Gilbert (p. 98). You may also f<strong>in</strong>d it helpful to review your read<strong>in</strong>g on<br />
C. <strong>elegans</strong> early development from Ch. 5.<br />
<strong>Problem</strong> Statement<br />
Several labs, beg<strong>in</strong>n<strong>in</strong>g <strong>in</strong> the 1980s, began <strong>in</strong>vestigat<strong>in</strong>g <strong>in</strong>ductive <strong>in</strong>teractions that lead to<br />
differentiation of the anterior-most cell from the four-cell embryo, AB.a, and its posterior sister<br />
cell, AB.p. These two cells give rise to cells of the pharynx, a mesodermal structure. Normally,<br />
AB.a generates cells that form the anterior pharynx, while AB.p normally gives rise to cells<br />
that make the posterior pharynx. These two types of cells express different prote<strong>in</strong>s and<br />
differentiate quite differently. There are several papers that you are free to consult. However, it is<br />
not necessary to read these papers to answer the questions on pages 2-3.Papers that may be<br />
relevant <strong>in</strong>clude the follow<strong>in</strong>g, which can be downloaded from Learn@UW:<br />
Priess, J. and Thomson, N. (1987). Cellular <strong>in</strong>teractions <strong>in</strong> early C. <strong>elegans</strong> <strong>embryos</strong>. Cell 48:241-50.<br />
Mello, C.C., Draper, B.W., and Priess, J.R. (1994). The maternal genes apx-1 and glp-1 and<br />
establishment of dorsal-ventral polarity <strong>in</strong> the early C. <strong>elegans</strong> embryo. Cell 77:95-106.<br />
Mango, S.E., Thorpe, C.J., Mart<strong>in</strong>, P.R., Chamberla<strong>in</strong>, S.H., and Bowerman, B. (1994). Two<br />
maternal genes, apx-1 and pie-1, are required to dist<strong>in</strong>guish the fates of equivalent blastomeres<br />
<strong>in</strong> the early Caenorhabditis <strong>elegans</strong> embryo. Development 120:2305-15.<br />
Evans, T.C., Crittenden, S.L., Kodoyianni, V., and Kimble, J. (1994). Translational control of<br />
maternal glp-1 mRNA establishes an asymmetry <strong>in</strong> the C. <strong>elegans</strong> embryo. Cell 77:183-94.
Zoo <strong>470</strong> – 2013 – <strong>Problem</strong> <strong>Set</strong> <strong>#2</strong> Page 2<br />
Name:________________________________ Student Number:__________________<br />
If you worked <strong>in</strong> a group, other collaborators: _____________________________________________<br />
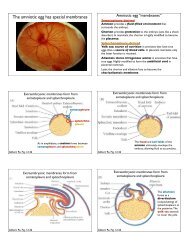
1. Test<strong>in</strong>g how AB.a and AB.p differentiate. Recall that there<br />
are four cells <strong>in</strong> the C. <strong>elegans</strong> embryo: AB.a, AB.p, EMS, and<br />
P2, whose spatial relationships are shown correctly at the right<br />
(anterior is to the left <strong>in</strong> the diagram).<br />
a. Jim Priess, Craig Mello, and Bruce Bowerman developed ways to<br />
manipulate blastomeres <strong>in</strong> the early embryo. They found they<br />
could move cells around by push<strong>in</strong>g them with a blunt needle, so that AB.a could be moved to lie <strong>in</strong><br />
the position normally occupied by AB.p. They also developed ways to remove cells from the embryo<br />
by suck<strong>in</strong>g them out of the eggshell with a pipette.<br />
a. (4 po<strong>in</strong>ts) Jim believed that P2 normally sends an <strong>in</strong>ductive signal to the AB daughter it is<br />
touch<strong>in</strong>g that leads that AB daughter to form posterior pharynx. Us<strong>in</strong>g the simple blastomere<br />
manipulations Jim developed, describe one experiment you could perform to show P2 contact is<br />
sufficient to <strong>in</strong>duce either AB daughter cell to form posterior pharynx.<br />
Mov<strong>in</strong>g P2 so that it touches AB.a <strong>in</strong>stead of AB.p should lead to the follow<strong>in</strong>g: AB.a should now<br />
make posterior pharynx. S<strong>in</strong>ce the question only asks for sufficiency. However, if such contact is<br />
<strong>in</strong>deed necessary, then AB.p, if it no longer touch<strong>in</strong>g P2, would not be expected to make posterior<br />
pharynx, and <strong>in</strong>stead would make anterior pharynx.<br />
b. (4 po<strong>in</strong>ts) Craig Mello and Jim Priess created the embryo below on the right (b). A normal embryo is<br />
shown for comparison (a).<br />
If Jim’s hypothesis is correct, will this embryo make more or less anterior pharynx than normal?<br />
Circle the correct answer:<br />
Embryo b will make more the same less anterior pharynx than normal<br />
Clearly state your reason<strong>in</strong>g: Contact with P2 is needed for posterior pharynx. Therefore, s<strong>in</strong>ce<br />
neither AB daughter can touch P2 <strong>in</strong> this case (see the picture), neither should make posterior<br />
pharynx, and therefore an excess of anterior pharynx should result. THis is <strong>in</strong> fact what happened<br />
with this embryo; Jim and Craig followed it until became a much more advanced embryo, and looked<br />
posterior vs. anterior pharynx.<br />
2. apx-1, glp-1, and pharynx differentiation<br />
a. (2 po<strong>in</strong>ts) Craig and Bruce (work<strong>in</strong>g <strong>in</strong>dependently) showed that a gene called apx-1 (for anterior<br />
pharynx <strong>in</strong> excess) is required for posterior pharynx differentiation. apx-1 is a maternal effect gene. If a<br />
mother (hermaphrodite) worm has the genotype apx-1(-)/+ [+ = wild-type copy of the gene], i.e., it is a<br />
heterozygote, what percentage of its offspr<strong>in</strong>g will have defective pharynges [“pharynges” is the plural of<br />
“pharynx”!]?<br />
Percentage of offspr<strong>in</strong>g from apx-1(-)/+ with defective pharynges: _____0%________<br />
S<strong>in</strong>ce the mother is a heterozygote, and it is her genotype that matters (apx-1 is materal effect), all<br />
of the offspr<strong>in</strong>g will appear normal.
Zoo <strong>470</strong> – 2013 – <strong>Problem</strong> <strong>Set</strong> <strong>#2</strong> Page 3<br />
2. (cont.)<br />
b. (4 po<strong>in</strong>ts) Please refer to p. 98 of Gilbert for <strong>in</strong>formation on the Notch/Delta pathway. Craig<br />
showed, us<strong>in</strong>g a special mutant <strong>in</strong> the glp-1 gene, that glp-1 is needed for correct differentiation of<br />
AB.p. glp-1 encodes a Notch prote<strong>in</strong>. Craig went on to show that apx-1 encodes a Delta family<br />
prote<strong>in</strong>. You are now work<strong>in</strong>g <strong>in</strong> Bruce Bowerman’s lab, where techniques were developed for<br />
isolat<strong>in</strong>g blastomeres and recomb<strong>in</strong><strong>in</strong>g them, as we discussed for Wnt/Frizzled signal<strong>in</strong>g <strong>in</strong> class.<br />
You are eager to try blastomere recomb<strong>in</strong>ations us<strong>in</strong>g cells lack<strong>in</strong>g either apx-1 or glp-1 function.<br />
You assess anterior pharynx formation us<strong>in</strong>g an antibody that recognizes anterior pharynx.<br />
Based on your study of Notch and Delta, what would happen if an AB.p cell lack<strong>in</strong>g APX-1 prote<strong>in</strong><br />
were comb<strong>in</strong>ed with a P2 cell lack<strong>in</strong>g GLP-1 prote<strong>in</strong>? Clearly expla<strong>in</strong> your answer.<br />
APX-1 the Delta-like ligand; GLP-1 the Notch-like receptor. P2 normally needs to produce the ligand<br />
(signal), and AB.p normally needs the receptor to sense it. Therefore <strong>in</strong> this case there would a<br />
normal mount of anterior pharynx, s<strong>in</strong>ce AB.p doesn’t need the ligand, and P2 doesn’t need the<br />
receptor.<br />
3. Translational control of glp-1<br />
a. (1 po<strong>in</strong>t) Tom Evans, who was work<strong>in</strong>g <strong>in</strong> Judith Kimble’s lab here at UW at the time, showed that<br />
glp-1 mRNA is found everywhere <strong>in</strong> the four-cell embryo, but that the prote<strong>in</strong> is only found <strong>in</strong> two<br />
cells <strong>in</strong> the four-cell embryo. What technique did he use to assess mRNA localization?<br />
Technique: ___<strong>in</strong> situ hybridization__________________________<br />
b. (3 po<strong>in</strong>ts) Tom hypothesized that the glp-1 mRNA is subject to translational control. To see which<br />
part of the mRNA was <strong>in</strong>volved, he used molecular biology techniques to make a piece of DNA that<br />
encoded a reporter prote<strong>in</strong> he could detect (β-galactosidase) fused to DNA encod<strong>in</strong>g parts of the<br />
glp-1 mRNA. He then assessed whether the artificial mRNAs produced from this DNA, when<br />
<strong>in</strong>troduced <strong>in</strong>to <strong>embryos</strong>, were only translated <strong>in</strong> two cells. What part of the glp-1 gene would you<br />
predict would need to be attached to the β-galactosidase cod<strong>in</strong>g region to cause it to only be<br />
expressed <strong>in</strong> two cells? Expla<strong>in</strong> your answer.<br />
Part of the glp-1 gene required for translation of the fusion prote<strong>in</strong> only <strong>in</strong> two cells: 3’-UTR<br />
Reason<strong>in</strong>g: Variants of the follow<strong>in</strong>g rationale are acceptable:<br />
The 3’-UTR is required for translational regulators (prote<strong>in</strong>, miRNAs, etc.) to b<strong>in</strong>d to an mRNA and<br />
regulate its expression (the polyadenylation “signal” sequence is also there). S<strong>in</strong>ce this is the region<br />
that controls where the prote<strong>in</strong> is translated, it would have to be hooked up the cod<strong>in</strong>g region for βgalactosidase<br />
to get the restricted prote<strong>in</strong> expression pattern.<br />
c. (2 po<strong>in</strong>ts) Neither of the two cells <strong>in</strong> which GLP-1 prote<strong>in</strong> is normally found is EMS. Based on this<br />
<strong>in</strong>formation, what are the most likely cells that conta<strong>in</strong> GLP-1?<br />
Two cells at the four-cell stage that conta<strong>in</strong> GLP-1: _____AB.a and AB.p______<br />
AB.a and AB.p are both capable of respond<strong>in</strong>g to the P2 signal, so they each would be expected to<br />
have the receptor to receive the signal. The signal is APX-1/Delta, so they would each be expected<br />
to have GLP-1/Notch.