Molybdenum glass melting electrodes
Molybdenum glass melting electrodes
Molybdenum glass melting electrodes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
10<br />
Temperature [˚C]<br />
Application Information<br />
Sealing by <strong>glass</strong>ification<br />
For the installation of the electrode it is recommended to first push it into the <strong>glass</strong> melt and then pull it back into the electrode<br />
holder, while turning it continuously in a clockwise direction. Through cooling of the electrode holder, the <strong>glass</strong> layer solidifies,<br />
thus providing protection against diffusion. Before inserting an extension electrode, the cooling of the holder should be slightly<br />
reduced so as to promote <strong>melting</strong> of the solidified <strong>glass</strong> layer.<br />
Sufficient cooling<br />
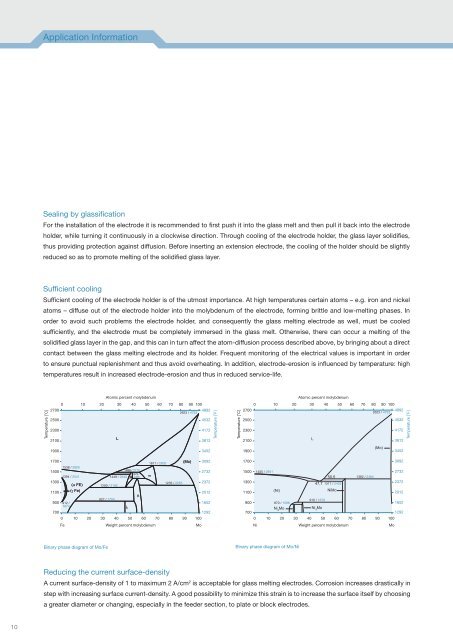
Sufficient cooling of the electrode holder is of the utmost importance. At high temperatures certain atoms – e.g. iron and nickel<br />
atoms – diffuse out of the electrode holder into the molybdenum of the electrode, forming brittle and low-<strong>melting</strong> phases. In<br />
order to avoid such problems the electrode holder, and consequently the <strong>glass</strong> <strong>melting</strong> electrode as well, must be cooled<br />
sufficiently, and the electrode must be completely immersed in the <strong>glass</strong> melt. Otherwise, there can occur a <strong>melting</strong> of the<br />
solidified <strong>glass</strong> layer in the gap, and this can in turn affect the atom-diffusion process described above, by bringing about a direct<br />
contact between the <strong>glass</strong> <strong>melting</strong> electrode and its holder. Frequent monitoring of the electrical values is important in order<br />
to ensure punctual replenishment and thus avoid overheating. In addition, electrode-erosion is influenced by temperature: high<br />
temperatures result in increased electrode-erosion and thus in reduced service-life.<br />
0 0<br />
2700 2700<br />
Temperature [˚C]<br />
2500 2500<br />
2300 2300<br />
2100 2100<br />
1900 1900<br />
1700 1700<br />
1538 / 2800 1538 / 2800<br />
1500 1500<br />
1394 / 2541 1394 / 2541<br />
1300 1300<br />
1100 1100<br />
900 912 900/<br />
1674<br />
700 700<br />
912 /<br />
1674<br />
Atomic Atomic percent percent molybdenum molybdenum<br />
10 10 20 20 30 3040 4050 50 60 60 70 80 70 90 80 100 90 100<br />
4892 4892<br />
2623 / 4753 2623 / 4753<br />
4532 4532<br />
1611 / 2932 1611 / 2932<br />
1488 / 2710 1488 / 2710<br />
1370 / 1370 /<br />
1449 / 2640 1449 / 2640 2498 2498<br />
1235 / 2255 1235 / 2255<br />
1200 / 2192 1200 / 2192<br />
927 / 1700 927 / 1700<br />
0 010 1020 2030 3040 4050 5060 6070 7080 809090 100 100<br />
Fe Fe Weight Weight percent percent molybdenum molybdenum<br />
Mo Mo<br />
Binary phase diagram of Mo/Fe<br />
Temperature [˚F]<br />
4172 4172<br />
3812 3812<br />
3452 3452<br />
3092 3092<br />
2732 2732<br />
2372 2372<br />
2012 2012<br />
1652 1652<br />
1292 1292<br />
Temperature [˚F]<br />
Temperature [˚C]<br />
0 0<br />
2700 2700<br />
Temperature [˚C]<br />
2500 2500<br />
2300 2300<br />
2100 2100<br />
1900 1900<br />
1700 1700<br />
1500 1500 1455 / 2651 1455 / 2651<br />
1300 1300<br />
1100 1100<br />
900<br />
700<br />
900<br />
700<br />
Atomic Atomic percent percent molybdenum molybdenum<br />
10 10 20 20 30 3040 4050 50 60 60 70 80 70 90 80 100 90 100<br />
4892 4892<br />
2623 / 4753 2623 / 4753<br />
4532 4532<br />
870 / 1598 870 / 1598<br />
1362 / 2484 1362 / 2484<br />
1317 / 2403 1317 / 2403<br />
910 / 1670 910 / 1670<br />
0 010 1020 2030 3040 4050 5060 6070 7080 809090 100 100<br />
Ni Ni Weight Weight percent percent molybdenum molybdenum<br />
Mo Mo<br />
Binary phase diagram of Mo/Ni<br />
Temperature [˚F]<br />
4172 4172<br />
3812 3812<br />
3452 3452<br />
3092 3092<br />
2732 2732<br />
2372 2372<br />
2012 2012<br />
1652 1652<br />
1292 1292<br />
Reducing the current surface-density<br />
A current surface-density of 1 to maximum 2 A/cm2 is acceptable for <strong>glass</strong> <strong>melting</strong> <strong>electrodes</strong>. Corrosion increases drastically in<br />
step with increasing surface current-density. A good possibility to minimize this strain is to increase the surface itself by choosing<br />
a greater diameter or changing, especially in the feeder section, to plate or block <strong>electrodes</strong>.<br />
Temperature [˚F]