Amphetamine and Related Drugs
Amphetamine and Related Drugs
Amphetamine and Related Drugs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Amphetamine</strong> <strong>and</strong><br />
<strong>Related</strong> <strong>Drugs</strong><br />
4/27/2009 ١
Narcotics of Natural Origin<br />
-Opium Opium<br />
-Morphine Morphine<br />
-Codeine Codeine<br />
-Thebaine Thebaine<br />
Semi-Synthetic Semi Synthetic Narcotics<br />
-Heroin Heroin<br />
-Hydromorphone<br />
Hydromorphone<br />
-Oxycodone Oxycodone<br />
-Hydrocodone<br />
Hydrocodone<br />
Synthetic Narcotics<br />
-Meperidine Meperidine<br />
-Dextropropoxyphene<br />
Dextropropoxyphene<br />
-Fentanyl Fentanyl<br />
-Pentazocine Pentazocine<br />
-Butorphanol<br />
Butorphanol<br />
Narcotics Treatment <strong>Drugs</strong><br />
-Methadone Methadone<br />
-LAAM LAAM<br />
-BuprenorphineNarcotics<br />
BuprenorphineNarcotics<br />
Narcotics<br />
4/27/2009 ٢
Cocaine<br />
<strong>Amphetamine</strong>s<br />
Methcathinone<br />
Methylphenidate<br />
Anorectic <strong>Drugs</strong><br />
Khat<br />
Stimulants<br />
4/27/2009 ٣
Depressants<br />
Barbiturates<br />
Benzodiazepines<br />
Flunitrazepam<br />
Gamma Hydroxybutyric Acid<br />
Paraldehyde<br />
Chloral Hydrate<br />
Glutethimide <strong>and</strong> Methaqualone<br />
Meprobamate<br />
4/27/2009 ٤
Marijuana<br />
Hashish<br />
Hashish Oil<br />
Cannabis<br />
4/27/2009 ٥
Hallucinogens<br />
LSD<br />
Psilocybin & Psilocyn <strong>and</strong> other Tryptamines<br />
Peyote & Mescaline<br />
New Hallucinogens<br />
MDMA (Ecstasy) <strong>and</strong> other<br />
Phenethylamines<br />
Phencyclidine <strong>and</strong> <strong>Related</strong> <strong>Drugs</strong><br />
Ketamine<br />
4/27/2009 ٦
Other Categories<br />
Inhalants like amyl <strong>and</strong> butyl nitrite, nitrous<br />
oxide <strong>and</strong> others<br />
Steroids<br />
4/27/2009 ٧
Drug Schedules: Schedule I<br />
• The drug or other substance has a high potential for<br />
abuse.<br />
• The drug or other substance has no currently<br />
accepted medical use in treatment in the United<br />
States.<br />
• There is a lack of accepted safety for use of the drug<br />
or other substance under medical supervision.<br />
• Examples of Schedule I substances include heroin,<br />
lysergic acid diethylamide (LSD), marijuana, <strong>and</strong><br />
methaqualone.<br />
methaqualone.<br />
4/27/2009 ٨
Schedule I amphetamine derivatives<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
2,5-Dimethoxy<br />
2,5 Dimethoxy-4-ethylamphetamine<br />
ethylamphetamine<br />
2,5-Dimethoxyamphetamine<br />
2,5 Dimethoxyamphetamine<br />
3,4,5-Trimethoxyamphetamine<br />
3,4,5 Trimethoxyamphetamine<br />
3,4-Methylenedioxyamphetamine<br />
3,4 Methylenedioxyamphetamine<br />
3,4-Methylenedioxymethamphetamine<br />
3,4 Methylenedioxymethamphetamine<br />
4-Bromo Bromo-2,5 2,5-dimethoxyamphetamine<br />
dimethoxyamphetamine<br />
4-Bromo Bromo-2,5 2,5-dimethoxyphenethylamine<br />
dimethoxyphenethylamine<br />
4-Methoxyamphetamine<br />
Methoxyamphetamine<br />
4-Methyl Methyl-2,5 2,5-dimethoxyamphetamine<br />
dimethoxyamphetamine<br />
5-Methoxy Methoxy-3,4 3,4-methylenedioxyamphetamine<br />
methylenedioxyamphetamine<br />
4/27/2009 ٩
Schedule II<br />
• The drug or other substance has a high potential for<br />
abuse.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States or a<br />
currently accepted medical use with severe<br />
restrictions.<br />
• Abuse of the drug or other substance may lead to<br />
severe psychological or physical dependence.<br />
•Examples •Examples<br />
of Schedule II include morphine,<br />
phencyclidine (PCP), cocaine, methadone, <strong>and</strong><br />
methamphetamine<br />
4/27/2009 ١٠
Schedule III<br />
• The drug or other substance has less potential for<br />
abuse than the drugs or other substances in schedules<br />
I <strong>and</strong> II.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States.<br />
• Abuse of the drug or other substance may lead to<br />
moderate or low physical dependence or high<br />
psychological dependence.<br />
• Anabolic steroids, codeine <strong>and</strong> hydrocodone with<br />
aspirin or Tylenol®, Tylenol , <strong>and</strong> some barbiturates are<br />
examples of Schedule III substances.<br />
4/27/2009 ١١
Schedule IV<br />
• The drug or other substance has a low potential for abuse<br />
relative to the drugs or other substances in Schedule III.<br />
• The drug or other substance has a currently accepted medical<br />
use in treatment in the United States.<br />
• Abuse of the drug or other substance may lead to limited<br />
physical dependence or psychological dependence relative to<br />
the drugs or other substances in Schedule III.<br />
•Examples •Examples<br />
of drugs included in schedule IV are Darvon®, Darvon ,<br />
Talwin®, Talwin , Equanil®, Equanil , Valium®, Valium , <strong>and</strong> Xanax®.<br />
Xanax<br />
4/27/2009 ١٢
Schedule V<br />
• The drug or other substance has a low potential for<br />
abuse relative to the drugs or other substances in<br />
Schedule IV.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States.<br />
• Abuse of the drug or other substances may lead to<br />
limited physical dependence or psychological<br />
dependence relative to the drugs or other substances<br />
in Schedule IV.<br />
•Cough •Cough<br />
medicines with codeine are examples of<br />
Schedule V drugs<br />
4/27/2009 ١٣
Most Common <strong>Amphetamine</strong>s<br />
There are a large number of amphetamines which are<br />
controlled substances. Of these, the most commonly<br />
encountered in the forensic science laboratory are<br />
amphetamine (1), ( ), methylamphetamine (2), ), 3,4- 3,4<br />
methylenedioxyamphetamine (MDA) (3), ( ), 3,4- 3,4<br />
methylenedioxymethylamphetamine (MDMA) (4)<strong>and</strong> ( )<strong>and</strong><br />
3,4-methylenedioxyethylamphetamine 3,4 methylenedioxyethylamphetamine (MDEA) (5). ( ).<br />
In addition, there are a wide variety of structurally<br />
related analogues which can be synthesized.<br />
4/27/2009 ١٤
4/27/2009 ١٥
Some history<br />
<strong>Amphetamine</strong> was first marketed in the 1930s as<br />
Benzedrine®<br />
Benzedrine in an over-the over the-counter counter inhaler to treat<br />
nasal congestion. By 1937, amphetamine was<br />
available by prescription in tablet form <strong>and</strong> was used<br />
in the treatment of the sleeping disorder, narcolepsy,<br />
<strong>and</strong> the behavioral syndrome called minimal brain<br />
dysfunction, which today is called attention deficit<br />
hyperactivity disorder (ADHD). During World War<br />
II, amphetamine was widely used to keep the fighting<br />
men going <strong>and</strong> both dextroamphetamine<br />
(Dexedrine®) (Dexedrine ) <strong>and</strong> methamphetamine (Methedrine ( Methedrine®) )<br />
were readily available.<br />
4/27/2009 ١٦
As use of amphetamines spread, so did their<br />
abuse. In the 1960s, amphetamines became a<br />
perceived remedy for helping truckers to<br />
complete their long routes without falling<br />
asleep, for weight control, for helping athletes<br />
to perform better, <strong>and</strong> for treating mild<br />
depression. With experience, it became evident<br />
that the dangers of abuse of these drugs<br />
outweighed most of their therapeutic uses.<br />
4/27/2009 ١٧
Increased control measures were initiated in 1965<br />
with amendments to the federal food <strong>and</strong> drug laws to<br />
curb the black market in amphetamines. Many<br />
pharmaceutical amphetamine products were removed<br />
from the market including all injectable formulations,<br />
<strong>and</strong> doctors prescribed those that remained less<br />
freely. <strong>Amphetamine</strong> products presently marketed<br />
include generic <strong>and</strong> br<strong>and</strong> name amphetamine<br />
(Adderall Adderall®, , Dexedrine®,<br />
Dexedrine , Dextrostat®) Dextrostat ) <strong>and</strong> br<strong>and</strong><br />
name methamphetamine (Desoxyn ( Desoxyn®). ).<br />
4/27/2009 ١٨
To meet the ever-increasing ever increasing black market dem<strong>and</strong> for<br />
amphetamines, cl<strong>and</strong>estine laboratory production has<br />
mushroomed. Today, most amphetamines distributed<br />
to the black market are produced in cl<strong>and</strong>estine<br />
laboratories. Methamphetamine laboratories are, by<br />
far, the most frequently encountered cl<strong>and</strong>estine<br />
laboratories in the United States. The ease of<br />
cl<strong>and</strong>estine synthesis, combined with tremendous<br />
profits, has resulted in significant availability of illicit<br />
methamphetamine, especially on the West Coast,<br />
where abuse of this drug has increased dramatically<br />
in recent years.<br />
4/27/2009 ١٩
<strong>Amphetamine</strong>s are generally taken orally or<br />
injected. However, the addition of "ice," the<br />
slang name for crystallized methamphetamine<br />
hydrochloride, has promoted smoking as<br />
another mode of administration. Just as<br />
"crack" is smokable cocaine, "ice" is smokable<br />
methamphetamine. Methamphetamine, in all<br />
its forms, is highly addictive <strong>and</strong> toxic.<br />
4/27/2009 ٢٠
The effects of amphetamines, especially methamphetamine,<br />
are similar to cocaine, but their onset is slower <strong>and</strong> their<br />
duration is longer. In contrast to cocaine, which is quickly<br />
removed from the brain <strong>and</strong> is almost completely metabolized,<br />
methamphetamine remains in the central nervous system<br />
longer, <strong>and</strong> a larger percentage of the drug remains unchanged<br />
in the body, producing prolonged stimulant effects. Chronic<br />
abuse produces a psychosis (severe mental disorder), picking<br />
at the skin, <strong>and</strong> visual hallucinations. These psychotic<br />
symptoms can persist for months <strong>and</strong> even years after use of<br />
these drugs has ceased <strong>and</strong> may be related to their neurotoxic<br />
effects. Violent <strong>and</strong> erratic behavior is frequently seen among<br />
chronic abusers of amphetamines, especially<br />
methamphetamine.<br />
4/27/2009 ٢١
AMPHETAMINE<br />
Amfetamine<br />
Central Stimulant<br />
Synonyms. <strong>Amphetamine</strong>; Anfetamina; Racemic<br />
Dexedrine.<br />
Proprietary names. It is an ingredient of<br />
Biphetamine <strong>and</strong> Durophet.<br />
4/27/2009 ٢٢
A colourless, mobile, slowly volatile liquid. It<br />
absorbs carbon dioxide from the air forming a<br />
volatile carbonate. B.p. 200° 200 to 203°. 203<br />
Soluble 1 in 50 of water; soluble in ethanol<br />
chloroform <strong>and</strong> ether; readily soluble in acids<br />
Colour Tests.<br />
Liebermann's Test (sulfuric acid + nitrous acid)<br />
—red red–orange; orange; Marquis Test—orange<br />
Test orange→brown; brown;<br />
Ninhydrin—pink<br />
Ninhydrin pink–orange orange<br />
4/27/2009 ٢٣
Amfetamine Phosphate<br />
Synonyms. <strong>Amphetamine</strong> Phosphate; Benzpropaminum<br />
Phosphoricum; Monobasic Racemic <strong>Amphetamine</strong><br />
Phosphate.<br />
FW = 233.2<br />
A white crystalline powder with no characteristic<br />
melting point; it sinters at about 150° 150 <strong>and</strong><br />
decomposes at about 300°. 300<br />
Freely soluble in water; slightly soluble in ethanol;<br />
practically insoluble in benzene, chloroform, <strong>and</strong><br />
ether.<br />
ether<br />
4/27/2009 ٢٤
Amfetamine Sulfate<br />
Synonyms. <strong>Amphetamine</strong> Sulfate; Phenaminum; Phenopromini<br />
Sulfas; Phenylaminopropanum Racemicum Sulfuricum;<br />
Psychedrinum.<br />
Proprietary names. Benzedrine; Centramina. It is an ingredient of<br />
Adderall, Epipropane, <strong>and</strong> Ortenal.<br />
FW = 368.5<br />
A white crystalline powder. M.p. above 300°, 300 , with<br />
decomposition.<br />
Soluble 1 in 9 of water <strong>and</strong> 1 in 515 of ethanol; practically<br />
insoluble in chloroform <strong>and</strong> ether.<br />
ether<br />
4/27/2009 ٢٥
Disposition in the Body.<br />
Readily absorbed after oral or rectal administration;<br />
rapidly distributed extravascularly <strong>and</strong> taken up, to<br />
some extent, by red blood cells. The main metabolic<br />
reaction is oxidative deamination to form<br />
phenylacetone, which is then oxidised to benzoic acid<br />
<strong>and</strong> conjugated with glycine to form hippuric acid;<br />
minor reactions include aromatic hydroxylation to<br />
form 4–hydroxyamfetamine 4 hydroxyamfetamine (an active metabolite), β-<br />
hydroxylation to form norephedrine<br />
(phenylpropanolamine), <strong>and</strong> N-oxidation oxidation to form a<br />
hydroxylamine derivative.<br />
4/27/2009 ٢٦
4/27/2009 ٢٧
Excretion of amfetamine is markedly dependent on urinary<br />
pH, being greatly increased in acid urine. After large doses,<br />
amfetamine may be detected in urine for several days. Under<br />
uncontrolled urinary pH conditions, about 30% of the dose is<br />
excreted unchanged in the urine in 24 h <strong>and</strong> a total of about<br />
90% of the dose is excreted in 3 to 4 days. The amount<br />
excreted unchanged in 24 h may increase to 74% of the dose in<br />
acid urine <strong>and</strong> decrease to 1 to 4% in alkaline urine; under<br />
alkaline conditions, hippuric acid <strong>and</strong> benzoic acid account for<br />
about 50% of the urinary material. Under normal conditions 16<br />
to 28% is excreted as hippuric acid, about 4% as<br />
benzoylglucuronide, 2 to 4% as 4–hydroxyamfetamine, 4 hydroxyamfetamine, <strong>and</strong><br />
about 2% as norephedrine in 24 h; small amounts of<br />
conjugated 4–hydroxynorephedrine 4 hydroxynorephedrine <strong>and</strong> phenylacetone are<br />
also excreted. No elimination in the faeces has been detected.<br />
4/27/2009 ٢٨
Therapeutic concentration<br />
After normal therapeutic doses the plasma<br />
concentration is usually below 0.1 mg/L. However,<br />
continued use of amfetamine may cause addiction,<br />
<strong>and</strong> ingestion of 10 times the usual therapeutic dose is<br />
common among addicts; in such cases the plasma<br />
concentration may be up to 3 mg/L.<br />
After a single oral dose containing 10 mg of<br />
amfetamine to 4 subjects, peak plasma concentrations<br />
of about 0.02 mg/L were attained<br />
4/27/2009 ٢٩
Steady–state Steady state blood concentrations of 2 to<br />
3 mg/L were observed in a regular user who<br />
ingested about 1 g a day.<br />
The intravenous administration of 160 mg of<br />
amfetamine to a regular user resulted in a<br />
plasma concentration of 0.59 mg/L after 1 h.<br />
4/27/2009 ٣٠
Toxicity<br />
The estimated minimum lethal dose in non–addicted<br />
non addicted<br />
adults is 200 mg. Toxic effects may be produced with<br />
blood concentrations of 0.2 to 3 mg/L, <strong>and</strong> fatalities<br />
with concentrations greater than 0.5 mg/L. Death<br />
from overdosage is comparatively rare.<br />
In a fatality caused by intravenous administration of<br />
amfetamine, the following postmortem tissue<br />
concentrations were reported: blood 41 mg/L, liver<br />
23 μg/g, g/g, urine 39 mg/L.<br />
4/27/2009 ٣١
Half–life. Half life.<br />
Plasma half–life, half life, 4 to 8 h when the urine is acidic <strong>and</strong><br />
about 12 h in subjects whose urinary pH values are<br />
uncontrolled.<br />
Volume of distribution.<br />
About 3 to 4 L/kg.<br />
Dose.<br />
20 to 100 mg of amfetamine sulfate daily has been<br />
used in the treatment of narcolepsy.<br />
4/27/2009 ٣٢
Methylenedioxymethamfetamine<br />
Stimulant, Hallucinogen<br />
Synonyms. Adam; Clarity; E; Ecstasy; Elaine;<br />
Essence; Euphoria; MDM; MDMA; 3,4- 3,4<br />
Methylenedioxymetamfetamine; 3,4– 3,4<br />
methylenedioxymethylamphetamine; Stacy; X;<br />
XTC.<br />
4/27/2009 ٣٣
Street names<br />
Several tablets <strong>and</strong> capsules have been in circulation<br />
over the years, containing varying amounts of<br />
MDMA <strong>and</strong> other phenethylamines, recognisable by<br />
logos on tablets or colours of capsules. Some<br />
examples of tablets or capsules include: Apples;<br />
Beans; Baby slits; Brownies; Burgers; Crowns;<br />
Dennis the Menaces; Diamond Whites; Disco<br />
biscuits; Dollars; Doves; Love Doves; Mitsubishis;<br />
Mitsies; New Yorkers; Pink Calis; Rhubarb &<br />
Custards; Tangos; White Doves.<br />
4/27/2009 ٣٤
N,α-Dimethyl Dimethyl–1,3,benzodioxole<br />
1,3,benzodioxole–5–<br />
ethanamine<br />
FW =193.2<br />
A viscous, colourless oil. B.p. 100° 100<br />
to 110°. 110 .<br />
4/27/2009 ٣٥
Methylenedioxymethamfetamine<br />
Hydrochloride<br />
FW = 229.75<br />
A white to off–white off white crystalline powder with a<br />
bitter taste. M.p. 147° 147 to 148° 148 for crystals from<br />
isopropanol/n–hexane; isopropanol/ hexane; M.p. 152° 152 to 153° 153 for<br />
crystals from isopropan-2-ol/ether.<br />
isopropan ol/ether.<br />
4/27/2009 ٣٦
Colour Test.<br />
Marquis Reagent—black Reagent black with dark purple.<br />
Thin–layer Thin layer Chromatography.<br />
System TA—Rf TA Rf 0.33; system TB—Rf TB Rf 0.24;<br />
system TE—Rf TE Rf 0.39; system TF—Rf TF Rf 0.20;<br />
system TAE—Rf TAE Rf 0.08; system TAJ—Rf TAJ Rf 0.03;<br />
system TAK—Rf TAK Rf 0.17; system TAL—Rf TAL Rf 0.57.<br />
4/27/2009 ٣٧
Disposition in the Body.<br />
It is absorbed into the blood stream after ingestion <strong>and</strong><br />
excreted in urine, the majority of the dose unchanged (65%<br />
within 3 days). Metabolism occurs by a number of routes: N-<br />
demethylation of the parent compound to 3,4– 3,4<br />
methylenedioxyamfetamine (MDA) (7%) with further O-<br />
demethylation to 3,4–dihydroxymethamfetamine 3,4 dihydroxymethamfetamine (HHMA)<br />
<strong>and</strong> 3,4–dihydroxyamfetamine 3,4 dihydroxyamfetamine (HHA). Both HHMA <strong>and</strong><br />
HHA are subsequently O-methylated methylated mainly to 4–hydroxy 4 hydroxy–3–<br />
methoxymetamfetamine (HMMA) <strong>and</strong> 4–hydroxy 4 hydroxy–3–<br />
methoxyamfetamine (HMA). These four metabolites are<br />
excreted in the urine as the conjugated glucuronide or sulfate<br />
metabolites.<br />
4/27/2009 ٣٨
Therapeutic concentration<br />
8 healthy male volunteers, aged between 21 <strong>and</strong> 31<br />
years old, were administered a 75 mg dose of<br />
MDMA. The mean peak plasma concentration was<br />
0.13 mg/L after 1.8 h. Mean peak plasma<br />
concentrations of the metabolite, 3,4– 3,4<br />
methylenedioxyamfetamine (MDA), were 7.8 μg/L g/L<br />
approximately 5 h after administration.<br />
4/27/2009 ٣٩
After the administration of a single oral dose of<br />
1.5 mg/kg body weight MDMA to 2 patients, plasma<br />
<strong>and</strong> urine samples were collected over periods of 9<br />
<strong>and</strong> 22 h, respectively. Peak plasma concentrations of<br />
MDMA <strong>and</strong> MDA were 331 μg/L g/L after 2 h <strong>and</strong><br />
15 μg/L g/L after 6.3 h, respectively. Peak concentrations<br />
of 28.1 μg/L g/L MDMA in urine appeared after 21.5 h.<br />
Up to 2.3 μg/L g/L MDA, 35.1 μg/L g/L HMMA, <strong>and</strong><br />
2.1 μg/L g/L HMA were measured within 16 to 21.5 h,<br />
also in urine.<br />
4/27/2009 ٤٠
Toxicity<br />
Fatalities with doses of 300 mg have been<br />
reported. Capable of causing severe toxicity<br />
<strong>and</strong> the pattern of acute toxicity is due to the<br />
circumstances in which it is misused. A lethal<br />
concentration of 0.35 to 0.50 mg/L in serum<br />
has been noted although some overdose cases<br />
report concentrations 10 times this amount,<br />
without fatality.<br />
4/27/2009 ٤١
Half–life. Half life.<br />
About 6 to 7 h.<br />
Clearance.<br />
The mean total clearance of MDMA for a 75 mg dose<br />
is 86.9 L/h.<br />
Protein binding<br />
About 65%<br />
Dose.<br />
The usual dose is between 80 <strong>and</strong> 200 mg (more<br />
often 100 to 150 mg).<br />
4/27/2009 ٤٢
Metamfetamine<br />
Central Stimulant<br />
Synonyms. d-Deoxyephedrine;<br />
Deoxyephedrine;<br />
Desoxyephedrine; Methamphetamine;<br />
Methylamfetamine; methylamphetamine;<br />
Phenylmethylaminopropane.<br />
Note.<br />
Metamfetamine in a smokeable form has been<br />
known as Crank, Crystal, Crystal meth, Ice,<br />
meth, <strong>and</strong> Speed.<br />
4/27/2009 ٤٣
Proprietary name. name Norodin<br />
FW =149.2<br />
A clear, colourless, slowly volatile, mobile<br />
liquid. Mass per mL 0.921 to 0.922 g. B.p.<br />
about 214°. 214<br />
Slightly soluble in water; miscible with<br />
ethanol, chloroform, <strong>and</strong> ether.<br />
4/27/2009 ٤٤
Metamfetamine Hydrochloride<br />
Proprietary names. names Amphedroxyn; Desfedrin;<br />
Desoxyfed; Desoxyn; Destim; Doxephrin; Drinalfa;<br />
Gerobit; Hiropon; Isophen; Isophen;<br />
Madrine; Methampex;<br />
Methedrine; Methylisomyn; Pervitin;<br />
Soxysympamine; Syndrox; Tonedron.<br />
FW =185.7<br />
White crystals or crystalline powder. M.p. 170° 170 to<br />
175°. 175<br />
Soluble 1 in 2 of water, 1 in 4 of ethanol, <strong>and</strong> 1 in 5<br />
of chloroform; practically insoluble in ether.<br />
4/27/2009 ٤٥
Colour Test.<br />
Marquis Test—orange.<br />
Test orange.<br />
Thin–layer Thin layer Chromatography.<br />
System TA—Rf TA Rf 0.31; system TB—Rf TB Rf 0.28; system<br />
TC—Rf TC Rf 13; system TE—Rf TE Rf 0.42; system TL—Rf TL Rf<br />
0.05; system TAE—Rf TAE Rf 0.09; system TAF—Rf TAF Rf 0.63;<br />
system TAJ—Rf TAJ Rf 0.00; system TAK—Rf TAK Rf 0.03; system<br />
TAL—Rf TAL Rf 0.45. (Dragendorff spray, positive;<br />
acidified iodoplatinate solution, positive; Marquis<br />
reagent, brown; ninhydrin spray, positive; acidified<br />
potassium permanganate solution, positive.)<br />
4/27/2009 ٤٦
Disposition in the Body<br />
Readily absorbed after oral administration. About<br />
70% of a dose is excreted in the urine in 24 h. Under<br />
normal conditions, up to 43% of a dose is excreted as<br />
unchanged drug, up to 15% as 4– 4<br />
hydroxymetamfetamine, <strong>and</strong> about 5% as<br />
amfetamine, amfetamine,<br />
the major active metabolite. A number<br />
of other metabolites have been identified. Excretion<br />
of unchanged drug is dependent on the urinary pH,<br />
being increased in acidic urine <strong>and</strong> greatly reduced<br />
(to about 2% of a dose) if the urine is alkaline<br />
4/27/2009 ٤٧
Following a single oral dose of 12.5 mg of<br />
metamfetamine hydrochloride to 10 subjects, a<br />
mean peak blood concentration of about<br />
0.02 mg/L was attained in about 2 h<br />
Toxicity<br />
The estimated minimum lethal dose is 1 g, but<br />
fatalities attributed to metamfetamine are rare.<br />
4/27/2009 ٤٨
Half–life Half life<br />
Plasma half–life, half life, about 9 h.<br />
Dose.<br />
2.5 to 25 mg of metamfetamine hydrochloride<br />
daily, by mouth; 15 to 20 mg IM, or 10 to<br />
15 mg IV.<br />
4/27/2009 ٤٩
Methylenedioxyethylamfetamine<br />
Stimulant, Hallucinogen<br />
Synonyms. N-Ethyl Ethyl–3,4 3,4–<br />
methylenedioxyphenylisopropylamine; Eve; MDE;<br />
MDEA; 3,4-Methylenedioxyethamphetamine; 3,4 Methylenedioxyethamphetamine; 3,4- 3,4<br />
Methylenedioxyethylamphetamine. Usually presented<br />
as Ecstasy.<br />
N-ethyl ethyl-α-methyl methyl–1,3 1,3–benzodioxole<br />
benzodioxole–5–ethanamine ethanamine<br />
FW =207.3<br />
4/27/2009 ٥٠
A viscous, colourless oil. B.p. is 0.2 is 0.2<br />
85° 85 to 95°<br />
95<br />
4/27/2009 ٥١
Methylenedioxyethylamfetamine<br />
Hydrochloride<br />
FW = 243.8<br />
A white to off–white off white crystalline powder with a<br />
bitter taste.<br />
4/27/2009 ٥٢
Disposition in the Body.<br />
It is absorbed into the blood stream after<br />
ingestion <strong>and</strong> excreted in urine, mainly as the<br />
parent drug (19%),<br />
methylenedioxyamfetamine (MDA, 28%) <strong>and</strong><br />
also 4–hydroxy 4 hydroxy–3–methoxyethylamfetamine<br />
methoxyethylamfetamine<br />
(HMEA, 32%).<br />
4/27/2009 ٥٣
Toxicity<br />
The estimated lethal dose is 0.5 g.<br />
In a 20-year 20 year-old old male whose death was<br />
attributed to injection of MDMA <strong>and</strong> MDEA,<br />
postmortem blood concentrations of 2.0 <strong>and</strong><br />
0.7 mg/L, respectively, were reported<br />
4/27/2009 ٥٤
Methylenedioxyamfetamine<br />
Hallucinogen<br />
Synonyms. MDA;<br />
Methylenedioxyamphetamine;<br />
Tenamfetamine; SKF-5. SKF 5.<br />
α-Methyl Methyl–1,3 1,3–benzodioxole<br />
benzodioxole–5–ethanamine ethanamine<br />
FW =179.2<br />
4/27/2009 ٥٥
4/27/2009 ٥٦
Why are adulterants <strong>and</strong> diluants<br />
added to a sample?<br />
There are a number of reasons for these additions.<br />
Adulterants, for example, caffeine <strong>and</strong> other<br />
pharmacologically active drugs, are added to either<br />
hide the lack of the desired drug, dilute it (<strong>and</strong> hence<br />
increase profit pro for the drug dealer), or add another<br />
type of effect to the drug mixture. Diluents are<br />
bulking agents <strong>and</strong> may include starch, talc, etc., <strong>and</strong><br />
are added to make the drug ‘go go further’. further . Some<br />
additives may be added to the powders, either to<br />
improve the flow ow properties of the tablets prior to the<br />
tableting process, or to impart a particular color.<br />
color<br />
4/27/2009 ٥٧
Qualitative Identification<br />
Identi cation of<br />
<strong>Amphetamine</strong>s<br />
A wide variety of types of sample may be<br />
expected by the forensic scientist. These<br />
include powdered drug material, tableted<br />
material <strong>and</strong> items likely to have traces of the<br />
drug samples present. From these items, the<br />
appropriate samples should be taken, described<br />
<strong>and</strong> subsequently forwarded for analysis .<br />
4/27/2009 ٥٨
Sampling <strong>and</strong> Physical Description of<br />
<strong>Amphetamine</strong>s<br />
Powder Samples<br />
If the samples are powdered materials, they should be sorted<br />
into groups, where the members of the groups cannot be<br />
distinguished from each other. Having achieved this, the items<br />
in each group should be counted <strong>and</strong> a good physical<br />
description prepared. This should include weight, color, odor<br />
<strong>and</strong> any other physical characteristic that the scientist<br />
considers to be important. Depending upon the number of<br />
items in the group, the following sampling strategy, based<br />
upon the United Nations Drug Control Program (UNDCP)<br />
recommendations, may be adopted. If there are between 1 <strong>and</strong><br />
10 items, all of them should be examined. If there are between<br />
10 <strong>and</strong> 100 items, then 10 items should be examined, while if<br />
there are more than 100 items, the square route of the number<br />
of items should be examined<br />
4/27/2009 ٥٩
Tableted Samples<br />
If the items are tableted, tableted,<br />
then a different approach is required.<br />
The items should be divided into visually indistinguishable<br />
groups <strong>and</strong> the number of items in each group should be<br />
counted, if necessary estimating the number from the mean<br />
weight of tablet <strong>and</strong> the total mass of the tablets, if very large large<br />
numbers are involved. A good physical description of the<br />
tablets should be made, including recording of the size, color,<br />
shape, logo <strong>and</strong> score marks (if present), while the ballistics<br />
(physical characteristics) of the tablet should be examined <strong>and</strong><br />
detailed. Photography is particularly helpful in this respect.<br />
This latter includes recording of all of the physical damage to<br />
the tablets which may be present.<br />
4/27/2009 ٦٠
Recording the ballistic features of tableted<br />
drug units<br />
Having recorded all of the physical data, the items to<br />
be examined chemically should be chosen. At the<br />
time of writing, there is no agreed best-practice<br />
best practice<br />
protocol for undertaking this <strong>and</strong> the analyst should<br />
work within the requirements of the judicial system in<br />
which he/she is operating. The theory applied to<br />
powdered amphetamines can be equally applied to<br />
amphetamine tablets. The latter should be sampled<br />
<strong>and</strong> presumptive tests, TLC <strong>and</strong> confirmatory<br />
con rmatory tests<br />
then carried out.<br />
4/27/2009 ٦١
If the samples are trace samples, that is, the drug<br />
present is likely to be easily contaminated, the<br />
approach should be that the operators, laboratory<br />
equipment <strong>and</strong> reagents to be used should be<br />
demonstrably free of drug residues prior to the<br />
commencement of the analysis. This can be achieved<br />
by washing the glassware, work surfaces <strong>and</strong><br />
operator’s operator s h<strong>and</strong>s with a small amount of methanol <strong>and</strong><br />
concentrating the dissolved materials. The same batch<br />
of solvent should be used for this procedure <strong>and</strong> for<br />
the analysis of the drug items themselves<br />
4/27/2009 ٦٢
Having carried out the control procedures, the item(s) item(s)<br />
to be examined should then be swabbed, individually,<br />
by using a clean swab soaked in a suitable solvent. A<br />
suitable solvent should freely dissolve the drug<br />
material, not cause decomposition of the drug or react<br />
with it, <strong>and</strong> be amenable to subsequent analytical<br />
procedures. It should be remembered that the<br />
swabbing process should leave enough material intact<br />
for a second <strong>and</strong> subsequent analysis. If the swab is<br />
not to be used immediately, it should be dried <strong>and</strong><br />
stored until needed.<br />
4/27/2009 ٦٣
Sample Homogenization<br />
Homogenization of powdered samples can be achieved by<br />
using a number of different methodologies. One of the best<br />
methods for samples likely to be encountered ‘on on the street’ street is<br />
the use of the ‘cone cone-<strong>and</strong> <strong>and</strong>-square square’ method. In this technique,<br />
the materials are mixed <strong>and</strong> the larger fragments reduced in<br />
size. The material is poured onto a flat at, , clean surface <strong>and</strong> then<br />
divided into four; opposite quarters are removed <strong>and</strong> the two<br />
remaining quarters recombined. The process is repeated until<br />
the desired sample size is achieved. For larger samples, a ‘core core’<br />
may be taken <strong>and</strong> then subjected to further homogenization by<br />
using this cone-<strong>and</strong> cone <strong>and</strong>-square square methodology.<br />
methodology<br />
4/27/2009 ٦٤
For tableted materials, homogenization is more<br />
problematic since if the tablet was<br />
homogenized, it would also be destroyed. For<br />
this reason, a sample is taken from the tablet<br />
<strong>and</strong> gently scraped from the dose form, away<br />
from any ballistic features which are likely to<br />
be of use in subsequent examinations. The<br />
powder is then thoroughly homogenized prior<br />
to testing .<br />
4/27/2009 ٦٥
Thin Layer Chromatography of<br />
<strong>Amphetamine</strong>s<br />
In order that the sample can be tested for the presence<br />
of amphetamines, a test solution must be prepared.<br />
The sample should be dissolved in a suitable solvent<br />
(methanol is commonly used) at a sample<br />
concentration of the order of 10 mgml −1 . This allows<br />
for the fact that many amphetamine samples at the<br />
‘street street level’ level are extremely weak, i.e. between 2 <strong>and</strong><br />
10% amphetamine in a matrix of adulterants <strong>and</strong><br />
diluants, diluants,<br />
giving a solution of approximately 0.2–1.0 0.2 1.0<br />
mgml −1 , namely a concentration at which the<br />
st<strong>and</strong>ards can be prepared.<br />
4/27/2009 ٦٦
The sample should be dissolved as fully as<br />
possible <strong>and</strong> centrifuged or filtered ltered to remove<br />
any solid particulates. A positive <strong>and</strong> negative<br />
control should also be prepared. The silica gel<br />
chromatographic plate should be marked up<br />
<strong>and</strong> the test solutions, plus the positive <strong>and</strong><br />
negative controls, placed on the plate <strong>and</strong> the<br />
latter allowed to develop in the chosen solvent<br />
system<br />
4/27/2009 ٦٧
Practical TLC Urine Tests<br />
The urine sample is not hydrolyzed for Test B. The<br />
urine sample is adjusted to pH 10 with potassium<br />
carbonate. Sodium chloride is also added <strong>and</strong> the<br />
mixture is extracted twice with chloroform. The<br />
chloroform phase each time is removed <strong>and</strong> filtered.<br />
The pooled chloroform extracts are washed with a<br />
weak solution of ammonium hydroxide. The washed<br />
chloroform is then extracted twice with 1 N sulfuric<br />
acid.<br />
acid<br />
4/27/2009 ٦٨
The pooled sulfuric acid extracts are then<br />
adjusted to pH 10 with concentrated potassium<br />
hydroxide <strong>and</strong> potassium carbonate. Sodium<br />
chloride is also added <strong>and</strong> the mixture is<br />
extracted twice with chloroform. The filtered<br />
<strong>and</strong> pooled chloroform is then carefully<br />
evaporated after the addition of one drop of a<br />
solution of 0.5% sulfuric acid in methanol.<br />
4/27/2009 ٦٩
The residue is redissolved in acetone methanol<br />
solution <strong>and</strong> applied to a T.L.C. plate for<br />
development. Test B solvent system contains<br />
methanol <strong>and</strong> ammonium hydoxide. hydoxide.<br />
Test B<br />
detects methadone, pethidine, pethidine,<br />
cocaine,<br />
amphetamine, methamphetamine, cyclazocine<br />
<strong>and</strong> D-propoxyphene<br />
D propoxyphene. . Many other organic<br />
bases would be extracted by this procedure <strong>and</strong><br />
appear on the T.L.C. plate.<br />
4/27/2009 ٧٠
Urine analyzed by Test B shows an increase in<br />
background with the age of urine which partially<br />
interferes with the location of the spots after T.L.C.<br />
<strong>Amphetamine</strong>, methamphetamine, pethidine <strong>and</strong><br />
methadone are more labile compounds than morphine<br />
<strong>and</strong> codeine <strong>and</strong> more susceptible to decomposition<br />
<strong>and</strong> chemical change during storage or during testing.<br />
testing<br />
4/27/2009 ٧١
Procedure for Test B<br />
Measure 20 ml of urine into a 50 ml glass- glass<br />
stoppered centrifuhe tube. Add 1 g of<br />
potassium carbonate to adjust the pH to 10.<br />
Add 4 g of sodium chloride. Add the salts<br />
using a powder funnel <strong>and</strong> measuring spoons.<br />
Shake to dissolve the salts.<br />
Add 20 ml of chloroform <strong>and</strong> shake for 5<br />
minutes <strong>and</strong> centrifuge.<br />
4/27/2009 ٧٢
Aspirate off the lower chloroform layer <strong>and</strong> filter<br />
into another tube.<br />
Add 20 ml of chloroform for a second extraction.<br />
Shake for 5 minutes <strong>and</strong> centrifuge.<br />
Aspirate off the lower chloroform layer <strong>and</strong> filter<br />
into the second tube.<br />
Wash the filtered pooled chloroform with 10 ml of<br />
pH 9 aqueous ammonium hydroxide solution as<br />
follows: shake for 5 minutes <strong>and</strong> centrifuge <strong>and</strong><br />
aspirate off <strong>and</strong> discard the upper wash phase.<br />
phase<br />
4/27/2009 ٧٣
Add 10 ml of 1 N sulfuric acid to the tube, shake for<br />
5 minutes <strong>and</strong> centrifuge. Aspirate off the upper<br />
acid phase <strong>and</strong> tranfer it to a third tube.<br />
Repeat the extraction with another 10 ml portion of<br />
1 N sulfuric acid <strong>and</strong> pool with the first extraction<br />
in the third tube. Discard the lower chloroform<br />
phase.<br />
To the acid phase in the third tube add 16 N<br />
potassium hydroxide dropwise (about 1.3 ml) to<br />
adjust the pH to about 7. Add 1 g of potassium<br />
carbonate to adjust the pH to 10. Add 4 g of sodium<br />
chlorides <strong>and</strong> shake to dissolve the salts.<br />
4/27/2009 ٧٤
Add 20 ml chloroform <strong>and</strong> shake for 5 minutes <strong>and</strong><br />
centrifuge. Aspirate the lower chloroform phase <strong>and</strong><br />
filter into a 50 ml beaker.<br />
Repeat the extraction with a second 20 ml of<br />
chloroform <strong>and</strong> aspirate off <strong>and</strong> discard the upper<br />
aqueous phase. Decant <strong>and</strong> filter the chloroform<br />
phase into the beaker. Add 3 ml of chloroform wash<br />
to the tube <strong>and</strong> filter into the beaker.<br />
4/27/2009 ٧٥
Add one drop of 0.5 % sulfuric acid in<br />
methanol to the pooled chloroform in the<br />
beaker.<br />
Evaporate carefully to near dryness in the<br />
vacuum oven at a temperature of 90 oC <strong>and</strong> a<br />
vacuum of 10 p.s.i. p.s.i.<br />
Remove the beaker <strong>and</strong><br />
allow the final few drops of solvent to air<br />
dry.<br />
4/27/2009 ٧٦
Transfer the residue to a 3 ml<br />
microcentrifuge tube using small portion (0.5<br />
ml) of 1:1 acetone-methanol. acetone methanol. Again<br />
evaporate at a slow boil to near dryness in<br />
the vacuum oven maintained at 10 p.s.i, p.s.i,<br />
<strong>and</strong><br />
60 oC. 4/27/2009 ٧٧
Remove a thin-layer thin layer plate from the desiccator<br />
just before it is to be spotted. Spot the sample<br />
residues, procedure controls <strong>and</strong> reference<br />
compounds on a thin-layer thin layer plate on the sample<br />
application line located 2.5 cm <strong>and</strong> parallel to<br />
the bottom edge of the plate.<br />
4/27/2009 ٧٨
Dissolve the residues in 20 µl l of 1:1 acetone- acetone<br />
methanol <strong>and</strong> spot the dissolved sample from<br />
the micro centrifuge tubes using a 10 µl l<br />
microsyringe. microsyringe.<br />
Repeat the spotting twice adding<br />
solvent each time to insure that all of the<br />
dissolved residue is transferred. Apply 30µg 30 g of<br />
methadone, 60 µg g each of amphetamine <strong>and</strong><br />
methamphetamine toward the center of the<br />
application line .<br />
4/27/2009 ٧٩
Place the spotted plated into the developing<br />
tank containing 3 ml of conc. ammonium<br />
hydroxide in 200 ml of methanol which has<br />
equilibrated for 10 minutes. Allow the<br />
development to proceed until there is about<br />
14 cm of front movement in about 30<br />
minutes.<br />
4/27/2009 ٨٠
Remove the developed plate <strong>and</strong> allow it to air<br />
dry for about one hour. Examine the plate<br />
under ultraviolet light for absorbing or<br />
fluorescent spots <strong>and</strong> circle the spots on the<br />
uncoated side of the plate using a china<br />
marking pencil. Spray with Dragendorff's<br />
Reagent <strong>and</strong> make notes of spots, colors <strong>and</strong><br />
intensities. Then spray with potassium<br />
iodoplatinate reagent <strong>and</strong> repeat the<br />
observations .<br />
4/27/2009 ٨١
Examine the batch of plates making<br />
appropriate comparisons. Spray the plates<br />
within two hours after development <strong>and</strong> read<br />
<strong>and</strong> interpret them as the sprays are applied<br />
<strong>and</strong> again 10 minutes later.<br />
4/27/2009 ٨٢
Methamphetamine (Methedrine ( Methedrine) ) 44(B)<br />
<strong>Amphetamine</strong> (Benzedrine) 54(B)<br />
Solvent System for Test B - Mixture of 200 ml<br />
of methanol <strong>and</strong> 3 ml of concentrated<br />
ammonium hydroxide.<br />
4/27/2009 ٨٣
The ammonia is added to achieve a process known as<br />
ion suppression. suppression.<br />
By converting the drugs to their<br />
free base forms, their polarities are reduced. This is<br />
because the nitrogen atom does not carry a positive<br />
charge in basic solution. The latter reduces the<br />
problem of (TLC) tailing, improves the mass transfer<br />
properties between the stationary <strong>and</strong> mobile phases,<br />
<strong>and</strong> thus improves the chromatographic quality.<br />
4/27/2009 ٨٤
In addition, MDA, MDMA <strong>and</strong> MDEA give rise to<br />
purple, orange/red <strong>and</strong> orange/red products,<br />
respectively. At each of the visualization stages, the<br />
retardation factor (or relative front) (Rf ( Rf) ) values of the<br />
visualized compounds should be calculated by using<br />
the following equation:<br />
Distance moved by the analyte of interest<br />
Rf = -------------------------------------------------------<br />
Distance moved by the solvent front<br />
4/27/2009 ٨٥
The Rf values of the unknowns are compared to those<br />
of the st<strong>and</strong>ards <strong>and</strong> if the data cannot be<br />
discriminated then a suggested match is called.<br />
Although when using this combination of<br />
presumptive tests <strong>and</strong> TLC it is possible to<br />
discriminate within this group of compounds, due to<br />
the extremely large number of amphetamines<br />
available, it is necessary to carry out a confirmatory<br />
con rmatory<br />
analytical technique. The foremost of these, for<br />
amphetamine identification<br />
identi cation, , is gas chromatography–<br />
chromatography<br />
mass spectrometry (GC–MS (GC MS)<br />
4/27/2009 ٨٦
Thin–layer Thin layer Chromatographic Systems<br />
for <strong>Amphetamine</strong><br />
System TA—Rf TA Rf 43; system TB—Rf TB Rf 20;<br />
system TC—Rf TC Rf 09; system TE—Rf TE Rf 43; system<br />
TL—Rf TL Rf 18; system TAE—Rf TAE Rf 12; system<br />
TAF—Rf TAF Rf 75. (Dragendorff spray, positive;<br />
FPN reagent, pink; acidified iodoplatinate<br />
solution, positive; Marquis reagent, brown;<br />
ninhydrin spray, positive; acidified potassium<br />
permanganate solution, positive). positive).<br />
4/27/2009 ٨٧
System TA<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Methanol:strong ammonia<br />
solution (100:1.5).<br />
Reference compounds: compounds:<br />
Atropine Rf 18,<br />
Codeine Rf 33, Chlorprothixene Rf 56,<br />
Diazepam Rf 75.<br />
4/27/2009 ٨٨
Colour test<br />
The Marquis test gives an orange colour for both<br />
amfetamine <strong>and</strong> metamfetamine.<br />
Thin layer chromatography<br />
TA: amfetamine Rf = 0.43, metamfetamine Rf = 0.31.<br />
TB: amfetamine Rf = 0.15, metamfetamine Rf = 0.28.<br />
Visualisation: acidified iodoplatinate solution.<br />
4/27/2009 ٨٩
System TB<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Cyclohexane:toluene<br />
Cyclohexane: toluene:diethylamine<br />
diethylamine (75:15:10).<br />
Reference compounds: compounds:<br />
Codeine Rf 06,<br />
Desipramine Rf 20, Prazepam Rf 36,<br />
Trimipramine Rf 62<br />
4/27/2009 ٩٠
System TC<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Chloroform:methanol (90:10).<br />
Reference compounds: compounds:<br />
Desipramine Rf 11,<br />
Physostigmine Rf 36, Trimipramine Rf 54,<br />
Lidocaine Rf 71.<br />
4/27/2009 ٩١
System TL<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Acetone.<br />
Reference compounds: compounds:<br />
Amitriptyline Rf 15,<br />
Procaine Rf 30, Papaverine Rf 47, Cinnarizine<br />
Rf 65.<br />
4/27/2009 ٩٢
System TAE<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick.<br />
Mobile phase: phase:<br />
Methanol.<br />
Reference compounds: compounds:<br />
Codeine Rf 20,<br />
Trimipramine Rf 36, Hydroxyzine Rf 56,<br />
Diazepam Rf 82.<br />
4/27/2009 ٩٣
System TAF<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick.<br />
Mobile phase: phase:<br />
Methanol:n-butanol Methanol: butanol (60:40) <strong>and</strong><br />
0.1 mol/L NaBr.<br />
Reference compounds: compounds:<br />
Codeine Rf 22,<br />
Diphenhydramine Rf 48, Quinine Rf 65,<br />
Diazepam Rf 85 .<br />
4/27/2009 ٩٤
Location reagents for systems TA, TB<br />
<strong>and</strong> TC<br />
Ninhydrin spray<br />
Spray the plate with the reagent <strong>and</strong> then heat<br />
in an oven at 100° 100 for 5 min. Violet or pink<br />
spots are given by primary amines <strong>and</strong> yellow<br />
colours<br />
Ninhydrin Spray: add 0.5 g of ninhydrin to<br />
10 mL of hydrochloric acid <strong>and</strong> dilute to<br />
100 mL with acetone. Prepare daily.<br />
4/27/2009 ٩٥
FPN reagent<br />
Red or brown-red brown red spots are given by<br />
phenothiazines <strong>and</strong> blue spots by<br />
dibenzazepines. This reagent may be used to<br />
overspray a plate which has been previously<br />
sprayed with ninhydrin spray.<br />
FPN Reagent: mix together 5 mL of ferric<br />
chloride solution, 45 mL of a 20% w/w<br />
solution of perchloric acid, <strong>and</strong> 50 mL of a<br />
50% v/v solution of nitric acid.<br />
4/27/2009 ٩٦
Dragendorff spray<br />
Yellow, orange, red-orange, red orange, or brown-orange brown orange spots<br />
are given by tertiary alkaloids. This reagent may be<br />
used to overspray a plate which has been previously<br />
sprayed with ninhydrin spray <strong>and</strong> FPN spray.<br />
Dragendorff Spray: (a) mix together 2 g of bismuth<br />
subnitrate, subnitrate,<br />
25 mL of acetic acid, <strong>and</strong> 100 mL of<br />
water; (b) dissolve 40 g of potassium iodide in<br />
100 mL of water. Mix together 10 mL of (a), 10 mL<br />
of (b), 20 mL of acetic acid, <strong>and</strong> 100 mL of water.<br />
Prepare every 2 days.<br />
4/27/2009 ٩٧
Acidified iodoplatinate solution<br />
Violet, blue-violet, blue violet, grey-violet, grey violet, or brown-violet brown violet spots<br />
on a pink background are given by tertiary amines<br />
<strong>and</strong> quaternary ammonium compounds. Primary <strong>and</strong><br />
secondary amines give dirtier colours. This solution<br />
may be used to overspray a plate which has<br />
previously been sprayed with ninhydrin spray, FPN<br />
reagent <strong>and</strong> Dragendorff spray.<br />
Iodoplatinate Solution, Acidified: add 5 mL of<br />
hydrochloric acid to 100 mL of iodoplatinate<br />
solution.<br />
4/27/2009 ٩٨
M<strong>and</strong>elin’s M<strong>and</strong>elin s reagent<br />
This reagent is preferably poured onto the<br />
plate because of the danger of spraying<br />
concentrated acid. Many different colours are<br />
given with a variety of drugs<br />
M<strong>and</strong>elin's Reagent: dissolve 0.5 g of<br />
ammonium vanadate in 1.5 mL of water <strong>and</strong><br />
dilute to 100 mL with sulfuric acid. Filter the<br />
solution through glass wool.<br />
4/27/2009 ٩٩
Marquis reagent<br />
This reagent is preferably poured onto the<br />
plate because of the danger of spraying<br />
concentrated acid. Black or violet spots are<br />
given by alkaloids related to morphine. Many<br />
different colours are given with a variety of<br />
drugs<br />
Marquis Reagent: mix 1 mL of<br />
formaldehyde solution with 9 mL of sulfuric<br />
acid. Prepare daily.<br />
4/27/2009 ١٠٠
Acidified potassium permanganate<br />
solution<br />
Yellow-brown Yellow brown spots on a violet background<br />
are given by drugs with unsaturated aliphatic<br />
bonds. bonds<br />
Potassium Permanganate Solution,<br />
Acidified: a 1% solution of potassium<br />
permanganate in 0.25 M sulfuric acid.<br />
4/27/2009 ١٠١
Gas Chromatography.<br />
System GA—amfetamine GA amfetamine RI 1125, amfetamine-TFA amfetamine TFA RI 1095,<br />
amfetamine-PFP amfetamine PFP RI 1330, amfetamine-TMS amfetamine TMS RI 1190,<br />
amfetamine-AC amfetamine AC RI 1501, art (formyl) RI 1100, M (3-OH (3 OH-)-<br />
PFP2 RI 1520, M (3-OH (3 OH-)-TMS2 TMS2 RI 1850, M (3-OH (3 OH-)-AC2 AC2<br />
RI 1930, M (4-OH (4 OH-) ) RI 1480, M (4-OH (4 OH-)-AC2 AC2 RI 1900, M<br />
(3,4–di (3,4 di-OH OH-)-AC3 AC3 RI 2150, M (OH-methoxy<br />
(OH methoxy-) ) RI 1465, M<br />
(OH-methoxy<br />
(OH methoxy-)-AC2 AC2 RI 2065, M (desamino–oxo<br />
(desamino oxo-OH OH-)-AC AC<br />
RI 1520, M (desamino–oxo<br />
(desamino oxo-OH OH-methoxy methoxy-) ) RI 1510, M<br />
(desamino–oxo<br />
(desamino oxo-OH OH-methoxy methoxy-)-AC AC RI 1600, M (desamino– (desamino<br />
oxo–di oxo di-OH OH-)-AC2 AC2 RI 1735; system GB—amfetamine GB amfetamine RI<br />
1150; art (formyl) RI 1142; system GC—RI GC RI 1536; system<br />
GF—RI GF RI 1315; system GAK—retention GAK retention time 4.9 min.<br />
4/27/2009 ١٠٢
Column: DB-5 DB 5 fused silica (30 m × 0.25 mm<br />
i.d., 0.25 μm m film thickness). Column<br />
temperature: 70° 70 for 1 min, ramp to 100° 100 at<br />
30°/min, 30 /min, <strong>and</strong> to 270° 270 at 10°/min. 10 /min. Injector<br />
temperature: 280°. 280 . Carrier gas: helium, flow<br />
rate 0.8 mL/min. MS detection. Retention<br />
time: 6.5 min.<br />
4/27/2009 ١٠٣
High Performance Liquid<br />
Chromatography.<br />
System HA—k HA k 0.9; system HB—k HB k 8.48;<br />
system HC—k HC k 0.98; system HX—RI HX RI 244;<br />
system HAA—retention HAA retention time, 3.7 min; system<br />
HBC—retention HBC retention time 2.1 min; system HBD— HBD<br />
retention time 3.7 min.<br />
4/27/2009 ١٠٤
Column: Chiralcel OD-RH OD RH (150 × 2 mm i.d.,<br />
5 μm) m) at 35°. 35 . Mobile phase: phosphate–citrate<br />
phosphate citrate<br />
buffer (pH 4.0) with sodium<br />
hexafluorophosphate (0.3 M):acetonitrile<br />
(43:57), flow rate 0.1 mL/min. Fluorescence<br />
detection (λex=330 ( ex=330 nm, λem=440 em=440 nm).<br />
Retention time: 24.6 min.<br />
4/27/2009 ١٠٥
Infra–red Infra red Spectrum<br />
Principal peaks at wavenumbers 700, 740, 1495, 1090,<br />
1605, 825 cm−1<br />
cm<br />
4/27/2009 ١٠٦
Definitive De nitive Identification<br />
Identi cation of<br />
<strong>Amphetamine</strong>s<br />
GC–MS GC MS is the preferred method for the identification<br />
identi cation<br />
of amphetamines. The discussion below centres on<br />
the analysis of amphetamine itself, although the same<br />
principles can also be applied to other members of<br />
this class of drug. However, there are a number of<br />
problems associated with the gas chromatographic<br />
analysis of amphetamine. Being highly polar in<br />
nature, this compound is liable to poor<br />
chromatographic behaviour <strong>and</strong> tailing if the<br />
analytical instrument is not scrupulously clean<br />
4/27/2009 ١٠٧
4/27/2009 ١٠٨
4/27/2009 ١٠٩
Furthermore, the highly polar nature of the<br />
amino group results in sorption of<br />
amphetamine to the surfaces of the GC system<br />
components. This, coupled with the often low<br />
concentration of the amphetamine in the<br />
sample, results in the false impression that<br />
there is no amphetamine present in the<br />
specimen under investigation<br />
4/27/2009 ١١٠
In order to alleviate this problem,<br />
derivatization can be employed. One of the<br />
easiest processes, for the analysis of<br />
amphetamine, is to derivatize directly with<br />
carbon disulfide disul de <strong>and</strong> it is this method which<br />
finds nds wide application in the United Kingdom.<br />
For bulk <strong>and</strong> trace samples, this is achieved by<br />
dissolving the material<br />
4/27/2009 ١١١
4/27/2009 ١١٢
The reaction (see equation (2.1)) is a simple,<br />
pre-column pre column derivatization, derivatization,<br />
involving the amino<br />
group of the amphetamine <strong>and</strong> the CS2<br />
.Thisprocess Thisprocess reduces the polarity of the<br />
product, improving its chromatographic<br />
behaviour <strong>and</strong> hence the sensitivity of the<br />
method. In addition, it results in a molecule<br />
which produces characteristic fragments from<br />
the ionization process:<br />
4/27/2009 ١١٣
4/27/2009 ١١٤
Quantification<br />
Quanti cation of <strong>Amphetamine</strong>s<br />
Due to the nature of the compounds being<br />
considered <strong>and</strong> the need for derivatization,<br />
derivatization,<br />
GC–MS GC MS is not considered the best technique<br />
for sample quantification<br />
quanti cation<br />
4/27/2009 ١١٥
There are a number of difficulties dif culties encountered with<br />
quantification<br />
quanti cation after employing derivatization. derivatization.<br />
These<br />
include the fact because derivatization is another<br />
h<strong>and</strong>ling stage in the analytical process, there is<br />
always the risk of sample contamination.<br />
Furthermore, the assumption is made that the<br />
derivatization reactions are ‘complete complete’ <strong>and</strong> that the<br />
corresponding derivatives are stable for the period<br />
between derivative formation <strong>and</strong> analysis. Further<br />
factors are that dilutions need to be extremely<br />
accurate <strong>and</strong> precise to obtain reliable numerical data<br />
<strong>and</strong> that derivatization can potentially lead to<br />
increases in numerical errors for such data .<br />
4/27/2009 ١١٦
The amphetamines (st<strong>and</strong>ards <strong>and</strong> samples) should be<br />
dissolved in methanolic HCl (100 ml of methanol to<br />
which 175 µl l of concentrated HCl has been added). A<br />
range of st<strong>and</strong>ard solutions should be prepared in<br />
order to give a range of concentrations above <strong>and</strong><br />
below that which the street sample is thought to<br />
contain, remembering that the latter may only contain<br />
between 0 <strong>and</strong> 5 wt% amphetamine. If necessary, the<br />
materials (particularly the case samples) should be<br />
sonicated <strong>and</strong>, following this, centrifuged to remove<br />
any solid materials. The supernatant is retained for<br />
subsequent analysis.<br />
4/27/2009 ١١٧
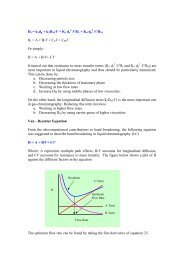
Having collected the data, a calibration curve should be<br />
plotted. Since amphetamine is frequently synthesized in dirty<br />
apparatus in ‘cl<strong>and</strong>estine cl<strong>and</strong>estine’ laboratories, it may not be possible<br />
to determine which salt form of the drug is present. The<br />
st<strong>and</strong>ard is generally supplied as the sulfate form, of the<br />
general formula (amphetamine sulfate). This means that for<br />
every gram of amphetamine sulfate, 73% will be present as the<br />
amphetamine free base. The calibration curve should be<br />
plotted as (UV detector) response against concentration of<br />
amphetamine free base<br />
4/27/2009 ١١٨
Derivatization<br />
4/27/2009 ١١٩
Derivatization results in good chromatographic<br />
behavior <strong>and</strong> provides compounds for which<br />
distinctive mass spectra can be obtained<br />
This method also has the advantage that the most<br />
common adulterant of amphetamine, namely caffeine,<br />
can also be identified identi ed in this system, <strong>and</strong> a suitable<br />
mass spectrum obtained As with all drug<br />
identification<br />
identi cation approaches, if the retention time data<br />
<strong>and</strong> mass spectra of the compounds match, then an<br />
identification<br />
identi cation can be inferred.<br />
4/27/2009 ١٢٠
Mass Spectra<br />
44 91 40 42 65 45 39 43 Amfetamine<br />
44 122 78 121 65 107 91 134<br />
Methoxyamfetamine<br />
44 136 51 135 77 42 78 45<br />
Methylenedioxyamfetamine<br />
44 138 122 137 121 91 78 45<br />
Methylthioamfetamine<br />
4/27/2009 ١٢١