Amphetamine and Related Drugs
Amphetamine and Related Drugs
Amphetamine and Related Drugs
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Amphetamine</strong> <strong>and</strong><br />
<strong>Related</strong> <strong>Drugs</strong><br />
4/27/2009 ١
Narcotics of Natural Origin<br />
-Opium Opium<br />
-Morphine Morphine<br />
-Codeine Codeine<br />
-Thebaine Thebaine<br />
Semi-Synthetic Semi Synthetic Narcotics<br />
-Heroin Heroin<br />
-Hydromorphone<br />
Hydromorphone<br />
-Oxycodone Oxycodone<br />
-Hydrocodone<br />
Hydrocodone<br />
Synthetic Narcotics<br />
-Meperidine Meperidine<br />
-Dextropropoxyphene<br />
Dextropropoxyphene<br />
-Fentanyl Fentanyl<br />
-Pentazocine Pentazocine<br />
-Butorphanol<br />
Butorphanol<br />
Narcotics Treatment <strong>Drugs</strong><br />
-Methadone Methadone<br />
-LAAM LAAM<br />
-BuprenorphineNarcotics<br />
BuprenorphineNarcotics<br />
Narcotics<br />
4/27/2009 ٢
Cocaine<br />
<strong>Amphetamine</strong>s<br />
Methcathinone<br />
Methylphenidate<br />
Anorectic <strong>Drugs</strong><br />
Khat<br />
Stimulants<br />
4/27/2009 ٣
Depressants<br />
Barbiturates<br />
Benzodiazepines<br />
Flunitrazepam<br />
Gamma Hydroxybutyric Acid<br />
Paraldehyde<br />
Chloral Hydrate<br />
Glutethimide <strong>and</strong> Methaqualone<br />
Meprobamate<br />
4/27/2009 ٤
Marijuana<br />
Hashish<br />
Hashish Oil<br />
Cannabis<br />
4/27/2009 ٥
Hallucinogens<br />
LSD<br />
Psilocybin & Psilocyn <strong>and</strong> other Tryptamines<br />
Peyote & Mescaline<br />
New Hallucinogens<br />
MDMA (Ecstasy) <strong>and</strong> other<br />
Phenethylamines<br />
Phencyclidine <strong>and</strong> <strong>Related</strong> <strong>Drugs</strong><br />
Ketamine<br />
4/27/2009 ٦
Other Categories<br />
Inhalants like amyl <strong>and</strong> butyl nitrite, nitrous<br />
oxide <strong>and</strong> others<br />
Steroids<br />
4/27/2009 ٧
Drug Schedules: Schedule I<br />
• The drug or other substance has a high potential for<br />
abuse.<br />
• The drug or other substance has no currently<br />
accepted medical use in treatment in the United<br />
States.<br />
• There is a lack of accepted safety for use of the drug<br />
or other substance under medical supervision.<br />
• Examples of Schedule I substances include heroin,<br />
lysergic acid diethylamide (LSD), marijuana, <strong>and</strong><br />
methaqualone.<br />
methaqualone.<br />
4/27/2009 ٨
Schedule I amphetamine derivatives<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
2,5-Dimethoxy<br />
2,5 Dimethoxy-4-ethylamphetamine<br />
ethylamphetamine<br />
2,5-Dimethoxyamphetamine<br />
2,5 Dimethoxyamphetamine<br />
3,4,5-Trimethoxyamphetamine<br />
3,4,5 Trimethoxyamphetamine<br />
3,4-Methylenedioxyamphetamine<br />
3,4 Methylenedioxyamphetamine<br />
3,4-Methylenedioxymethamphetamine<br />
3,4 Methylenedioxymethamphetamine<br />
4-Bromo Bromo-2,5 2,5-dimethoxyamphetamine<br />
dimethoxyamphetamine<br />
4-Bromo Bromo-2,5 2,5-dimethoxyphenethylamine<br />
dimethoxyphenethylamine<br />
4-Methoxyamphetamine<br />
Methoxyamphetamine<br />
4-Methyl Methyl-2,5 2,5-dimethoxyamphetamine<br />
dimethoxyamphetamine<br />
5-Methoxy Methoxy-3,4 3,4-methylenedioxyamphetamine<br />
methylenedioxyamphetamine<br />
4/27/2009 ٩
Schedule II<br />
• The drug or other substance has a high potential for<br />
abuse.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States or a<br />
currently accepted medical use with severe<br />
restrictions.<br />
• Abuse of the drug or other substance may lead to<br />
severe psychological or physical dependence.<br />
•Examples •Examples<br />
of Schedule II include morphine,<br />
phencyclidine (PCP), cocaine, methadone, <strong>and</strong><br />
methamphetamine<br />
4/27/2009 ١٠
Schedule III<br />
• The drug or other substance has less potential for<br />
abuse than the drugs or other substances in schedules<br />
I <strong>and</strong> II.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States.<br />
• Abuse of the drug or other substance may lead to<br />
moderate or low physical dependence or high<br />
psychological dependence.<br />
• Anabolic steroids, codeine <strong>and</strong> hydrocodone with<br />
aspirin or Tylenol®, Tylenol , <strong>and</strong> some barbiturates are<br />
examples of Schedule III substances.<br />
4/27/2009 ١١
Schedule IV<br />
• The drug or other substance has a low potential for abuse<br />
relative to the drugs or other substances in Schedule III.<br />
• The drug or other substance has a currently accepted medical<br />
use in treatment in the United States.<br />
• Abuse of the drug or other substance may lead to limited<br />
physical dependence or psychological dependence relative to<br />
the drugs or other substances in Schedule III.<br />
•Examples •Examples<br />
of drugs included in schedule IV are Darvon®, Darvon ,<br />
Talwin®, Talwin , Equanil®, Equanil , Valium®, Valium , <strong>and</strong> Xanax®.<br />
Xanax<br />
4/27/2009 ١٢
Schedule V<br />
• The drug or other substance has a low potential for<br />
abuse relative to the drugs or other substances in<br />
Schedule IV.<br />
• The drug or other substance has a currently accepted<br />
medical use in treatment in the United States.<br />
• Abuse of the drug or other substances may lead to<br />
limited physical dependence or psychological<br />
dependence relative to the drugs or other substances<br />
in Schedule IV.<br />
•Cough •Cough<br />
medicines with codeine are examples of<br />
Schedule V drugs<br />
4/27/2009 ١٣
Most Common <strong>Amphetamine</strong>s<br />
There are a large number of amphetamines which are<br />
controlled substances. Of these, the most commonly<br />
encountered in the forensic science laboratory are<br />
amphetamine (1), ( ), methylamphetamine (2), ), 3,4- 3,4<br />
methylenedioxyamphetamine (MDA) (3), ( ), 3,4- 3,4<br />
methylenedioxymethylamphetamine (MDMA) (4)<strong>and</strong> ( )<strong>and</strong><br />
3,4-methylenedioxyethylamphetamine 3,4 methylenedioxyethylamphetamine (MDEA) (5). ( ).<br />
In addition, there are a wide variety of structurally<br />
related analogues which can be synthesized.<br />
4/27/2009 ١٤
4/27/2009 ١٥
Some history<br />
<strong>Amphetamine</strong> was first marketed in the 1930s as<br />
Benzedrine®<br />
Benzedrine in an over-the over the-counter counter inhaler to treat<br />
nasal congestion. By 1937, amphetamine was<br />
available by prescription in tablet form <strong>and</strong> was used<br />
in the treatment of the sleeping disorder, narcolepsy,<br />
<strong>and</strong> the behavioral syndrome called minimal brain<br />
dysfunction, which today is called attention deficit<br />
hyperactivity disorder (ADHD). During World War<br />
II, amphetamine was widely used to keep the fighting<br />
men going <strong>and</strong> both dextroamphetamine<br />
(Dexedrine®) (Dexedrine ) <strong>and</strong> methamphetamine (Methedrine ( Methedrine®) )<br />
were readily available.<br />
4/27/2009 ١٦
As use of amphetamines spread, so did their<br />
abuse. In the 1960s, amphetamines became a<br />
perceived remedy for helping truckers to<br />
complete their long routes without falling<br />
asleep, for weight control, for helping athletes<br />
to perform better, <strong>and</strong> for treating mild<br />
depression. With experience, it became evident<br />
that the dangers of abuse of these drugs<br />
outweighed most of their therapeutic uses.<br />
4/27/2009 ١٧
Increased control measures were initiated in 1965<br />
with amendments to the federal food <strong>and</strong> drug laws to<br />
curb the black market in amphetamines. Many<br />
pharmaceutical amphetamine products were removed<br />
from the market including all injectable formulations,<br />
<strong>and</strong> doctors prescribed those that remained less<br />
freely. <strong>Amphetamine</strong> products presently marketed<br />
include generic <strong>and</strong> br<strong>and</strong> name amphetamine<br />
(Adderall Adderall®, , Dexedrine®,<br />
Dexedrine , Dextrostat®) Dextrostat ) <strong>and</strong> br<strong>and</strong><br />
name methamphetamine (Desoxyn ( Desoxyn®). ).<br />
4/27/2009 ١٨
To meet the ever-increasing ever increasing black market dem<strong>and</strong> for<br />
amphetamines, cl<strong>and</strong>estine laboratory production has<br />
mushroomed. Today, most amphetamines distributed<br />
to the black market are produced in cl<strong>and</strong>estine<br />
laboratories. Methamphetamine laboratories are, by<br />
far, the most frequently encountered cl<strong>and</strong>estine<br />
laboratories in the United States. The ease of<br />
cl<strong>and</strong>estine synthesis, combined with tremendous<br />
profits, has resulted in significant availability of illicit<br />
methamphetamine, especially on the West Coast,<br />
where abuse of this drug has increased dramatically<br />
in recent years.<br />
4/27/2009 ١٩
<strong>Amphetamine</strong>s are generally taken orally or<br />
injected. However, the addition of "ice," the<br />
slang name for crystallized methamphetamine<br />
hydrochloride, has promoted smoking as<br />
another mode of administration. Just as<br />
"crack" is smokable cocaine, "ice" is smokable<br />
methamphetamine. Methamphetamine, in all<br />
its forms, is highly addictive <strong>and</strong> toxic.<br />
4/27/2009 ٢٠
The effects of amphetamines, especially methamphetamine,<br />
are similar to cocaine, but their onset is slower <strong>and</strong> their<br />
duration is longer. In contrast to cocaine, which is quickly<br />
removed from the brain <strong>and</strong> is almost completely metabolized,<br />
methamphetamine remains in the central nervous system<br />
longer, <strong>and</strong> a larger percentage of the drug remains unchanged<br />
in the body, producing prolonged stimulant effects. Chronic<br />
abuse produces a psychosis (severe mental disorder), picking<br />
at the skin, <strong>and</strong> visual hallucinations. These psychotic<br />
symptoms can persist for months <strong>and</strong> even years after use of<br />
these drugs has ceased <strong>and</strong> may be related to their neurotoxic<br />
effects. Violent <strong>and</strong> erratic behavior is frequently seen among<br />
chronic abusers of amphetamines, especially<br />
methamphetamine.<br />
4/27/2009 ٢١
AMPHETAMINE<br />
Amfetamine<br />
Central Stimulant<br />
Synonyms. <strong>Amphetamine</strong>; Anfetamina; Racemic<br />
Dexedrine.<br />
Proprietary names. It is an ingredient of<br />
Biphetamine <strong>and</strong> Durophet.<br />
4/27/2009 ٢٢
A colourless, mobile, slowly volatile liquid. It<br />
absorbs carbon dioxide from the air forming a<br />
volatile carbonate. B.p. 200° 200 to 203°. 203<br />
Soluble 1 in 50 of water; soluble in ethanol<br />
chloroform <strong>and</strong> ether; readily soluble in acids<br />
Colour Tests.<br />
Liebermann's Test (sulfuric acid + nitrous acid)<br />
—red red–orange; orange; Marquis Test—orange<br />
Test orange→brown; brown;<br />
Ninhydrin—pink<br />
Ninhydrin pink–orange orange<br />
4/27/2009 ٢٣
Amfetamine Phosphate<br />
Synonyms. <strong>Amphetamine</strong> Phosphate; Benzpropaminum<br />
Phosphoricum; Monobasic Racemic <strong>Amphetamine</strong><br />
Phosphate.<br />
FW = 233.2<br />
A white crystalline powder with no characteristic<br />
melting point; it sinters at about 150° 150 <strong>and</strong><br />
decomposes at about 300°. 300<br />
Freely soluble in water; slightly soluble in ethanol;<br />
practically insoluble in benzene, chloroform, <strong>and</strong><br />
ether.<br />
ether<br />
4/27/2009 ٢٤
Amfetamine Sulfate<br />
Synonyms. <strong>Amphetamine</strong> Sulfate; Phenaminum; Phenopromini<br />
Sulfas; Phenylaminopropanum Racemicum Sulfuricum;<br />
Psychedrinum.<br />
Proprietary names. Benzedrine; Centramina. It is an ingredient of<br />
Adderall, Epipropane, <strong>and</strong> Ortenal.<br />
FW = 368.5<br />
A white crystalline powder. M.p. above 300°, 300 , with<br />
decomposition.<br />
Soluble 1 in 9 of water <strong>and</strong> 1 in 515 of ethanol; practically<br />
insoluble in chloroform <strong>and</strong> ether.<br />
ether<br />
4/27/2009 ٢٥
Disposition in the Body.<br />
Readily absorbed after oral or rectal administration;<br />
rapidly distributed extravascularly <strong>and</strong> taken up, to<br />
some extent, by red blood cells. The main metabolic<br />
reaction is oxidative deamination to form<br />
phenylacetone, which is then oxidised to benzoic acid<br />
<strong>and</strong> conjugated with glycine to form hippuric acid;<br />
minor reactions include aromatic hydroxylation to<br />
form 4–hydroxyamfetamine 4 hydroxyamfetamine (an active metabolite), β-<br />
hydroxylation to form norephedrine<br />
(phenylpropanolamine), <strong>and</strong> N-oxidation oxidation to form a<br />
hydroxylamine derivative.<br />
4/27/2009 ٢٦
4/27/2009 ٢٧
Excretion of amfetamine is markedly dependent on urinary<br />
pH, being greatly increased in acid urine. After large doses,<br />
amfetamine may be detected in urine for several days. Under<br />
uncontrolled urinary pH conditions, about 30% of the dose is<br />
excreted unchanged in the urine in 24 h <strong>and</strong> a total of about<br />
90% of the dose is excreted in 3 to 4 days. The amount<br />
excreted unchanged in 24 h may increase to 74% of the dose in<br />
acid urine <strong>and</strong> decrease to 1 to 4% in alkaline urine; under<br />
alkaline conditions, hippuric acid <strong>and</strong> benzoic acid account for<br />
about 50% of the urinary material. Under normal conditions 16<br />
to 28% is excreted as hippuric acid, about 4% as<br />
benzoylglucuronide, 2 to 4% as 4–hydroxyamfetamine, 4 hydroxyamfetamine, <strong>and</strong><br />
about 2% as norephedrine in 24 h; small amounts of<br />
conjugated 4–hydroxynorephedrine 4 hydroxynorephedrine <strong>and</strong> phenylacetone are<br />
also excreted. No elimination in the faeces has been detected.<br />
4/27/2009 ٢٨
Therapeutic concentration<br />
After normal therapeutic doses the plasma<br />
concentration is usually below 0.1 mg/L. However,<br />
continued use of amfetamine may cause addiction,<br />
<strong>and</strong> ingestion of 10 times the usual therapeutic dose is<br />
common among addicts; in such cases the plasma<br />
concentration may be up to 3 mg/L.<br />
After a single oral dose containing 10 mg of<br />
amfetamine to 4 subjects, peak plasma concentrations<br />
of about 0.02 mg/L were attained<br />
4/27/2009 ٢٩
Steady–state Steady state blood concentrations of 2 to<br />
3 mg/L were observed in a regular user who<br />
ingested about 1 g a day.<br />
The intravenous administration of 160 mg of<br />
amfetamine to a regular user resulted in a<br />
plasma concentration of 0.59 mg/L after 1 h.<br />
4/27/2009 ٣٠
Toxicity<br />
The estimated minimum lethal dose in non–addicted<br />
non addicted<br />
adults is 200 mg. Toxic effects may be produced with<br />
blood concentrations of 0.2 to 3 mg/L, <strong>and</strong> fatalities<br />
with concentrations greater than 0.5 mg/L. Death<br />
from overdosage is comparatively rare.<br />
In a fatality caused by intravenous administration of<br />
amfetamine, the following postmortem tissue<br />
concentrations were reported: blood 41 mg/L, liver<br />
23 μg/g, g/g, urine 39 mg/L.<br />
4/27/2009 ٣١
Half–life. Half life.<br />
Plasma half–life, half life, 4 to 8 h when the urine is acidic <strong>and</strong><br />
about 12 h in subjects whose urinary pH values are<br />
uncontrolled.<br />
Volume of distribution.<br />
About 3 to 4 L/kg.<br />
Dose.<br />
20 to 100 mg of amfetamine sulfate daily has been<br />
used in the treatment of narcolepsy.<br />
4/27/2009 ٣٢
Methylenedioxymethamfetamine<br />
Stimulant, Hallucinogen<br />
Synonyms. Adam; Clarity; E; Ecstasy; Elaine;<br />
Essence; Euphoria; MDM; MDMA; 3,4- 3,4<br />
Methylenedioxymetamfetamine; 3,4– 3,4<br />
methylenedioxymethylamphetamine; Stacy; X;<br />
XTC.<br />
4/27/2009 ٣٣
Street names<br />
Several tablets <strong>and</strong> capsules have been in circulation<br />
over the years, containing varying amounts of<br />
MDMA <strong>and</strong> other phenethylamines, recognisable by<br />
logos on tablets or colours of capsules. Some<br />
examples of tablets or capsules include: Apples;<br />
Beans; Baby slits; Brownies; Burgers; Crowns;<br />
Dennis the Menaces; Diamond Whites; Disco<br />
biscuits; Dollars; Doves; Love Doves; Mitsubishis;<br />
Mitsies; New Yorkers; Pink Calis; Rhubarb &<br />
Custards; Tangos; White Doves.<br />
4/27/2009 ٣٤
N,α-Dimethyl Dimethyl–1,3,benzodioxole<br />
1,3,benzodioxole–5–<br />
ethanamine<br />
FW =193.2<br />
A viscous, colourless oil. B.p. 100° 100<br />
to 110°. 110 .<br />
4/27/2009 ٣٥
Methylenedioxymethamfetamine<br />
Hydrochloride<br />
FW = 229.75<br />
A white to off–white off white crystalline powder with a<br />
bitter taste. M.p. 147° 147 to 148° 148 for crystals from<br />
isopropanol/n–hexane; isopropanol/ hexane; M.p. 152° 152 to 153° 153 for<br />
crystals from isopropan-2-ol/ether.<br />
isopropan ol/ether.<br />
4/27/2009 ٣٦
Colour Test.<br />
Marquis Reagent—black Reagent black with dark purple.<br />
Thin–layer Thin layer Chromatography.<br />
System TA—Rf TA Rf 0.33; system TB—Rf TB Rf 0.24;<br />
system TE—Rf TE Rf 0.39; system TF—Rf TF Rf 0.20;<br />
system TAE—Rf TAE Rf 0.08; system TAJ—Rf TAJ Rf 0.03;<br />
system TAK—Rf TAK Rf 0.17; system TAL—Rf TAL Rf 0.57.<br />
4/27/2009 ٣٧
Disposition in the Body.<br />
It is absorbed into the blood stream after ingestion <strong>and</strong><br />
excreted in urine, the majority of the dose unchanged (65%<br />
within 3 days). Metabolism occurs by a number of routes: N-<br />
demethylation of the parent compound to 3,4– 3,4<br />
methylenedioxyamfetamine (MDA) (7%) with further O-<br />
demethylation to 3,4–dihydroxymethamfetamine 3,4 dihydroxymethamfetamine (HHMA)<br />
<strong>and</strong> 3,4–dihydroxyamfetamine 3,4 dihydroxyamfetamine (HHA). Both HHMA <strong>and</strong><br />
HHA are subsequently O-methylated methylated mainly to 4–hydroxy 4 hydroxy–3–<br />
methoxymetamfetamine (HMMA) <strong>and</strong> 4–hydroxy 4 hydroxy–3–<br />
methoxyamfetamine (HMA). These four metabolites are<br />
excreted in the urine as the conjugated glucuronide or sulfate<br />
metabolites.<br />
4/27/2009 ٣٨
Therapeutic concentration<br />
8 healthy male volunteers, aged between 21 <strong>and</strong> 31<br />
years old, were administered a 75 mg dose of<br />
MDMA. The mean peak plasma concentration was<br />
0.13 mg/L after 1.8 h. Mean peak plasma<br />
concentrations of the metabolite, 3,4– 3,4<br />
methylenedioxyamfetamine (MDA), were 7.8 μg/L g/L<br />
approximately 5 h after administration.<br />
4/27/2009 ٣٩
After the administration of a single oral dose of<br />
1.5 mg/kg body weight MDMA to 2 patients, plasma<br />
<strong>and</strong> urine samples were collected over periods of 9<br />
<strong>and</strong> 22 h, respectively. Peak plasma concentrations of<br />
MDMA <strong>and</strong> MDA were 331 μg/L g/L after 2 h <strong>and</strong><br />
15 μg/L g/L after 6.3 h, respectively. Peak concentrations<br />
of 28.1 μg/L g/L MDMA in urine appeared after 21.5 h.<br />
Up to 2.3 μg/L g/L MDA, 35.1 μg/L g/L HMMA, <strong>and</strong><br />
2.1 μg/L g/L HMA were measured within 16 to 21.5 h,<br />
also in urine.<br />
4/27/2009 ٤٠
Toxicity<br />
Fatalities with doses of 300 mg have been<br />
reported. Capable of causing severe toxicity<br />
<strong>and</strong> the pattern of acute toxicity is due to the<br />
circumstances in which it is misused. A lethal<br />
concentration of 0.35 to 0.50 mg/L in serum<br />
has been noted although some overdose cases<br />
report concentrations 10 times this amount,<br />
without fatality.<br />
4/27/2009 ٤١
Half–life. Half life.<br />
About 6 to 7 h.<br />
Clearance.<br />
The mean total clearance of MDMA for a 75 mg dose<br />
is 86.9 L/h.<br />
Protein binding<br />
About 65%<br />
Dose.<br />
The usual dose is between 80 <strong>and</strong> 200 mg (more<br />
often 100 to 150 mg).<br />
4/27/2009 ٤٢
Metamfetamine<br />
Central Stimulant<br />
Synonyms. d-Deoxyephedrine;<br />
Deoxyephedrine;<br />
Desoxyephedrine; Methamphetamine;<br />
Methylamfetamine; methylamphetamine;<br />
Phenylmethylaminopropane.<br />
Note.<br />
Metamfetamine in a smokeable form has been<br />
known as Crank, Crystal, Crystal meth, Ice,<br />
meth, <strong>and</strong> Speed.<br />
4/27/2009 ٤٣
Proprietary name. name Norodin<br />
FW =149.2<br />
A clear, colourless, slowly volatile, mobile<br />
liquid. Mass per mL 0.921 to 0.922 g. B.p.<br />
about 214°. 214<br />
Slightly soluble in water; miscible with<br />
ethanol, chloroform, <strong>and</strong> ether.<br />
4/27/2009 ٤٤
Metamfetamine Hydrochloride<br />
Proprietary names. names Amphedroxyn; Desfedrin;<br />
Desoxyfed; Desoxyn; Destim; Doxephrin; Drinalfa;<br />
Gerobit; Hiropon; Isophen; Isophen;<br />
Madrine; Methampex;<br />
Methedrine; Methylisomyn; Pervitin;<br />
Soxysympamine; Syndrox; Tonedron.<br />
FW =185.7<br />
White crystals or crystalline powder. M.p. 170° 170 to<br />
175°. 175<br />
Soluble 1 in 2 of water, 1 in 4 of ethanol, <strong>and</strong> 1 in 5<br />
of chloroform; practically insoluble in ether.<br />
4/27/2009 ٤٥
Colour Test.<br />
Marquis Test—orange.<br />
Test orange.<br />
Thin–layer Thin layer Chromatography.<br />
System TA—Rf TA Rf 0.31; system TB—Rf TB Rf 0.28; system<br />
TC—Rf TC Rf 13; system TE—Rf TE Rf 0.42; system TL—Rf TL Rf<br />
0.05; system TAE—Rf TAE Rf 0.09; system TAF—Rf TAF Rf 0.63;<br />
system TAJ—Rf TAJ Rf 0.00; system TAK—Rf TAK Rf 0.03; system<br />
TAL—Rf TAL Rf 0.45. (Dragendorff spray, positive;<br />
acidified iodoplatinate solution, positive; Marquis<br />
reagent, brown; ninhydrin spray, positive; acidified<br />
potassium permanganate solution, positive.)<br />
4/27/2009 ٤٦
Disposition in the Body<br />
Readily absorbed after oral administration. About<br />
70% of a dose is excreted in the urine in 24 h. Under<br />
normal conditions, up to 43% of a dose is excreted as<br />
unchanged drug, up to 15% as 4– 4<br />
hydroxymetamfetamine, <strong>and</strong> about 5% as<br />
amfetamine, amfetamine,<br />
the major active metabolite. A number<br />
of other metabolites have been identified. Excretion<br />
of unchanged drug is dependent on the urinary pH,<br />
being increased in acidic urine <strong>and</strong> greatly reduced<br />
(to about 2% of a dose) if the urine is alkaline<br />
4/27/2009 ٤٧
Following a single oral dose of 12.5 mg of<br />
metamfetamine hydrochloride to 10 subjects, a<br />
mean peak blood concentration of about<br />
0.02 mg/L was attained in about 2 h<br />
Toxicity<br />
The estimated minimum lethal dose is 1 g, but<br />
fatalities attributed to metamfetamine are rare.<br />
4/27/2009 ٤٨
Half–life Half life<br />
Plasma half–life, half life, about 9 h.<br />
Dose.<br />
2.5 to 25 mg of metamfetamine hydrochloride<br />
daily, by mouth; 15 to 20 mg IM, or 10 to<br />
15 mg IV.<br />
4/27/2009 ٤٩
Methylenedioxyethylamfetamine<br />
Stimulant, Hallucinogen<br />
Synonyms. N-Ethyl Ethyl–3,4 3,4–<br />
methylenedioxyphenylisopropylamine; Eve; MDE;<br />
MDEA; 3,4-Methylenedioxyethamphetamine; 3,4 Methylenedioxyethamphetamine; 3,4- 3,4<br />
Methylenedioxyethylamphetamine. Usually presented<br />
as Ecstasy.<br />
N-ethyl ethyl-α-methyl methyl–1,3 1,3–benzodioxole<br />
benzodioxole–5–ethanamine ethanamine<br />
FW =207.3<br />
4/27/2009 ٥٠
A viscous, colourless oil. B.p. is 0.2 is 0.2<br />
85° 85 to 95°<br />
95<br />
4/27/2009 ٥١
Methylenedioxyethylamfetamine<br />
Hydrochloride<br />
FW = 243.8<br />
A white to off–white off white crystalline powder with a<br />
bitter taste.<br />
4/27/2009 ٥٢
Disposition in the Body.<br />
It is absorbed into the blood stream after<br />
ingestion <strong>and</strong> excreted in urine, mainly as the<br />
parent drug (19%),<br />
methylenedioxyamfetamine (MDA, 28%) <strong>and</strong><br />
also 4–hydroxy 4 hydroxy–3–methoxyethylamfetamine<br />
methoxyethylamfetamine<br />
(HMEA, 32%).<br />
4/27/2009 ٥٣
Toxicity<br />
The estimated lethal dose is 0.5 g.<br />
In a 20-year 20 year-old old male whose death was<br />
attributed to injection of MDMA <strong>and</strong> MDEA,<br />
postmortem blood concentrations of 2.0 <strong>and</strong><br />
0.7 mg/L, respectively, were reported<br />
4/27/2009 ٥٤
Methylenedioxyamfetamine<br />
Hallucinogen<br />
Synonyms. MDA;<br />
Methylenedioxyamphetamine;<br />
Tenamfetamine; SKF-5. SKF 5.<br />
α-Methyl Methyl–1,3 1,3–benzodioxole<br />
benzodioxole–5–ethanamine ethanamine<br />
FW =179.2<br />
4/27/2009 ٥٥
4/27/2009 ٥٦
Why are adulterants <strong>and</strong> diluants<br />
added to a sample?<br />
There are a number of reasons for these additions.<br />
Adulterants, for example, caffeine <strong>and</strong> other<br />
pharmacologically active drugs, are added to either<br />
hide the lack of the desired drug, dilute it (<strong>and</strong> hence<br />
increase profit pro for the drug dealer), or add another<br />
type of effect to the drug mixture. Diluents are<br />
bulking agents <strong>and</strong> may include starch, talc, etc., <strong>and</strong><br />
are added to make the drug ‘go go further’. further . Some<br />
additives may be added to the powders, either to<br />
improve the flow ow properties of the tablets prior to the<br />
tableting process, or to impart a particular color.<br />
color<br />
4/27/2009 ٥٧
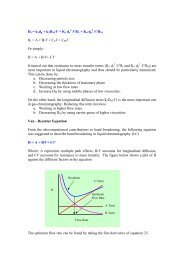
Qualitative Identification<br />
Identi cation of<br />
<strong>Amphetamine</strong>s<br />
A wide variety of types of sample may be<br />
expected by the forensic scientist. These<br />
include powdered drug material, tableted<br />
material <strong>and</strong> items likely to have traces of the<br />
drug samples present. From these items, the<br />
appropriate samples should be taken, described<br />
<strong>and</strong> subsequently forwarded for analysis .<br />
4/27/2009 ٥٨
Sampling <strong>and</strong> Physical Description of<br />
<strong>Amphetamine</strong>s<br />
Powder Samples<br />
If the samples are powdered materials, they should be sorted<br />
into groups, where the members of the groups cannot be<br />
distinguished from each other. Having achieved this, the items<br />
in each group should be counted <strong>and</strong> a good physical<br />
description prepared. This should include weight, color, odor<br />
<strong>and</strong> any other physical characteristic that the scientist<br />
considers to be important. Depending upon the number of<br />
items in the group, the following sampling strategy, based<br />
upon the United Nations Drug Control Program (UNDCP)<br />
recommendations, may be adopted. If there are between 1 <strong>and</strong><br />
10 items, all of them should be examined. If there are between<br />
10 <strong>and</strong> 100 items, then 10 items should be examined, while if<br />
there are more than 100 items, the square route of the number<br />
of items should be examined<br />
4/27/2009 ٥٩
Tableted Samples<br />
If the items are tableted, tableted,<br />
then a different approach is required.<br />
The items should be divided into visually indistinguishable<br />
groups <strong>and</strong> the number of items in each group should be<br />
counted, if necessary estimating the number from the mean<br />
weight of tablet <strong>and</strong> the total mass of the tablets, if very large large<br />
numbers are involved. A good physical description of the<br />
tablets should be made, including recording of the size, color,<br />
shape, logo <strong>and</strong> score marks (if present), while the ballistics<br />
(physical characteristics) of the tablet should be examined <strong>and</strong><br />
detailed. Photography is particularly helpful in this respect.<br />
This latter includes recording of all of the physical damage to<br />
the tablets which may be present.<br />
4/27/2009 ٦٠
Recording the ballistic features of tableted<br />
drug units<br />
Having recorded all of the physical data, the items to<br />
be examined chemically should be chosen. At the<br />
time of writing, there is no agreed best-practice<br />
best practice<br />
protocol for undertaking this <strong>and</strong> the analyst should<br />
work within the requirements of the judicial system in<br />
which he/she is operating. The theory applied to<br />
powdered amphetamines can be equally applied to<br />
amphetamine tablets. The latter should be sampled<br />
<strong>and</strong> presumptive tests, TLC <strong>and</strong> confirmatory<br />
con rmatory tests<br />
then carried out.<br />
4/27/2009 ٦١
If the samples are trace samples, that is, the drug<br />
present is likely to be easily contaminated, the<br />
approach should be that the operators, laboratory<br />
equipment <strong>and</strong> reagents to be used should be<br />
demonstrably free of drug residues prior to the<br />
commencement of the analysis. This can be achieved<br />
by washing the glassware, work surfaces <strong>and</strong><br />
operator’s operator s h<strong>and</strong>s with a small amount of methanol <strong>and</strong><br />
concentrating the dissolved materials. The same batch<br />
of solvent should be used for this procedure <strong>and</strong> for<br />
the analysis of the drug items themselves<br />
4/27/2009 ٦٢
Having carried out the control procedures, the item(s) item(s)<br />
to be examined should then be swabbed, individually,<br />
by using a clean swab soaked in a suitable solvent. A<br />
suitable solvent should freely dissolve the drug<br />
material, not cause decomposition of the drug or react<br />
with it, <strong>and</strong> be amenable to subsequent analytical<br />
procedures. It should be remembered that the<br />
swabbing process should leave enough material intact<br />
for a second <strong>and</strong> subsequent analysis. If the swab is<br />
not to be used immediately, it should be dried <strong>and</strong><br />
stored until needed.<br />
4/27/2009 ٦٣
Sample Homogenization<br />
Homogenization of powdered samples can be achieved by<br />
using a number of different methodologies. One of the best<br />
methods for samples likely to be encountered ‘on on the street’ street is<br />
the use of the ‘cone cone-<strong>and</strong> <strong>and</strong>-square square’ method. In this technique,<br />
the materials are mixed <strong>and</strong> the larger fragments reduced in<br />
size. The material is poured onto a flat at, , clean surface <strong>and</strong> then<br />
divided into four; opposite quarters are removed <strong>and</strong> the two<br />
remaining quarters recombined. The process is repeated until<br />
the desired sample size is achieved. For larger samples, a ‘core core’<br />
may be taken <strong>and</strong> then subjected to further homogenization by<br />
using this cone-<strong>and</strong> cone <strong>and</strong>-square square methodology.<br />
methodology<br />
4/27/2009 ٦٤
For tableted materials, homogenization is more<br />
problematic since if the tablet was<br />
homogenized, it would also be destroyed. For<br />
this reason, a sample is taken from the tablet<br />
<strong>and</strong> gently scraped from the dose form, away<br />
from any ballistic features which are likely to<br />
be of use in subsequent examinations. The<br />
powder is then thoroughly homogenized prior<br />
to testing .<br />
4/27/2009 ٦٥
Thin Layer Chromatography of<br />
<strong>Amphetamine</strong>s<br />
In order that the sample can be tested for the presence<br />
of amphetamines, a test solution must be prepared.<br />
The sample should be dissolved in a suitable solvent<br />
(methanol is commonly used) at a sample<br />
concentration of the order of 10 mgml −1 . This allows<br />
for the fact that many amphetamine samples at the<br />
‘street street level’ level are extremely weak, i.e. between 2 <strong>and</strong><br />
10% amphetamine in a matrix of adulterants <strong>and</strong><br />
diluants, diluants,<br />
giving a solution of approximately 0.2–1.0 0.2 1.0<br />
mgml −1 , namely a concentration at which the<br />
st<strong>and</strong>ards can be prepared.<br />
4/27/2009 ٦٦
The sample should be dissolved as fully as<br />
possible <strong>and</strong> centrifuged or filtered ltered to remove<br />
any solid particulates. A positive <strong>and</strong> negative<br />
control should also be prepared. The silica gel<br />
chromatographic plate should be marked up<br />
<strong>and</strong> the test solutions, plus the positive <strong>and</strong><br />
negative controls, placed on the plate <strong>and</strong> the<br />
latter allowed to develop in the chosen solvent<br />
system<br />
4/27/2009 ٦٧
Practical TLC Urine Tests<br />
The urine sample is not hydrolyzed for Test B. The<br />
urine sample is adjusted to pH 10 with potassium<br />
carbonate. Sodium chloride is also added <strong>and</strong> the<br />
mixture is extracted twice with chloroform. The<br />
chloroform phase each time is removed <strong>and</strong> filtered.<br />
The pooled chloroform extracts are washed with a<br />
weak solution of ammonium hydroxide. The washed<br />
chloroform is then extracted twice with 1 N sulfuric<br />
acid.<br />
acid<br />
4/27/2009 ٦٨
The pooled sulfuric acid extracts are then<br />
adjusted to pH 10 with concentrated potassium<br />
hydroxide <strong>and</strong> potassium carbonate. Sodium<br />
chloride is also added <strong>and</strong> the mixture is<br />
extracted twice with chloroform. The filtered<br />
<strong>and</strong> pooled chloroform is then carefully<br />
evaporated after the addition of one drop of a<br />
solution of 0.5% sulfuric acid in methanol.<br />
4/27/2009 ٦٩
The residue is redissolved in acetone methanol<br />
solution <strong>and</strong> applied to a T.L.C. plate for<br />
development. Test B solvent system contains<br />
methanol <strong>and</strong> ammonium hydoxide. hydoxide.<br />
Test B<br />
detects methadone, pethidine, pethidine,<br />
cocaine,<br />
amphetamine, methamphetamine, cyclazocine<br />
<strong>and</strong> D-propoxyphene<br />
D propoxyphene. . Many other organic<br />
bases would be extracted by this procedure <strong>and</strong><br />
appear on the T.L.C. plate.<br />
4/27/2009 ٧٠
Urine analyzed by Test B shows an increase in<br />
background with the age of urine which partially<br />
interferes with the location of the spots after T.L.C.<br />
<strong>Amphetamine</strong>, methamphetamine, pethidine <strong>and</strong><br />
methadone are more labile compounds than morphine<br />
<strong>and</strong> codeine <strong>and</strong> more susceptible to decomposition<br />
<strong>and</strong> chemical change during storage or during testing.<br />
testing<br />
4/27/2009 ٧١
Procedure for Test B<br />
Measure 20 ml of urine into a 50 ml glass- glass<br />
stoppered centrifuhe tube. Add 1 g of<br />
potassium carbonate to adjust the pH to 10.<br />
Add 4 g of sodium chloride. Add the salts<br />
using a powder funnel <strong>and</strong> measuring spoons.<br />
Shake to dissolve the salts.<br />
Add 20 ml of chloroform <strong>and</strong> shake for 5<br />
minutes <strong>and</strong> centrifuge.<br />
4/27/2009 ٧٢
Aspirate off the lower chloroform layer <strong>and</strong> filter<br />
into another tube.<br />
Add 20 ml of chloroform for a second extraction.<br />
Shake for 5 minutes <strong>and</strong> centrifuge.<br />
Aspirate off the lower chloroform layer <strong>and</strong> filter<br />
into the second tube.<br />
Wash the filtered pooled chloroform with 10 ml of<br />
pH 9 aqueous ammonium hydroxide solution as<br />
follows: shake for 5 minutes <strong>and</strong> centrifuge <strong>and</strong><br />
aspirate off <strong>and</strong> discard the upper wash phase.<br />
phase<br />
4/27/2009 ٧٣
Add 10 ml of 1 N sulfuric acid to the tube, shake for<br />
5 minutes <strong>and</strong> centrifuge. Aspirate off the upper<br />
acid phase <strong>and</strong> tranfer it to a third tube.<br />
Repeat the extraction with another 10 ml portion of<br />
1 N sulfuric acid <strong>and</strong> pool with the first extraction<br />
in the third tube. Discard the lower chloroform<br />
phase.<br />
To the acid phase in the third tube add 16 N<br />
potassium hydroxide dropwise (about 1.3 ml) to<br />
adjust the pH to about 7. Add 1 g of potassium<br />
carbonate to adjust the pH to 10. Add 4 g of sodium<br />
chlorides <strong>and</strong> shake to dissolve the salts.<br />
4/27/2009 ٧٤
Add 20 ml chloroform <strong>and</strong> shake for 5 minutes <strong>and</strong><br />
centrifuge. Aspirate the lower chloroform phase <strong>and</strong><br />
filter into a 50 ml beaker.<br />
Repeat the extraction with a second 20 ml of<br />
chloroform <strong>and</strong> aspirate off <strong>and</strong> discard the upper<br />
aqueous phase. Decant <strong>and</strong> filter the chloroform<br />
phase into the beaker. Add 3 ml of chloroform wash<br />
to the tube <strong>and</strong> filter into the beaker.<br />
4/27/2009 ٧٥
Add one drop of 0.5 % sulfuric acid in<br />
methanol to the pooled chloroform in the<br />
beaker.<br />
Evaporate carefully to near dryness in the<br />
vacuum oven at a temperature of 90 oC <strong>and</strong> a<br />
vacuum of 10 p.s.i. p.s.i.<br />
Remove the beaker <strong>and</strong><br />
allow the final few drops of solvent to air<br />
dry.<br />
4/27/2009 ٧٦
Transfer the residue to a 3 ml<br />
microcentrifuge tube using small portion (0.5<br />
ml) of 1:1 acetone-methanol. acetone methanol. Again<br />
evaporate at a slow boil to near dryness in<br />
the vacuum oven maintained at 10 p.s.i, p.s.i,<br />
<strong>and</strong><br />
60 oC. 4/27/2009 ٧٧
Remove a thin-layer thin layer plate from the desiccator<br />
just before it is to be spotted. Spot the sample<br />
residues, procedure controls <strong>and</strong> reference<br />
compounds on a thin-layer thin layer plate on the sample<br />
application line located 2.5 cm <strong>and</strong> parallel to<br />
the bottom edge of the plate.<br />
4/27/2009 ٧٨
Dissolve the residues in 20 µl l of 1:1 acetone- acetone<br />
methanol <strong>and</strong> spot the dissolved sample from<br />
the micro centrifuge tubes using a 10 µl l<br />
microsyringe. microsyringe.<br />
Repeat the spotting twice adding<br />
solvent each time to insure that all of the<br />
dissolved residue is transferred. Apply 30µg 30 g of<br />
methadone, 60 µg g each of amphetamine <strong>and</strong><br />
methamphetamine toward the center of the<br />
application line .<br />
4/27/2009 ٧٩
Place the spotted plated into the developing<br />
tank containing 3 ml of conc. ammonium<br />
hydroxide in 200 ml of methanol which has<br />
equilibrated for 10 minutes. Allow the<br />
development to proceed until there is about<br />
14 cm of front movement in about 30<br />
minutes.<br />
4/27/2009 ٨٠
Remove the developed plate <strong>and</strong> allow it to air<br />
dry for about one hour. Examine the plate<br />
under ultraviolet light for absorbing or<br />
fluorescent spots <strong>and</strong> circle the spots on the<br />
uncoated side of the plate using a china<br />
marking pencil. Spray with Dragendorff's<br />
Reagent <strong>and</strong> make notes of spots, colors <strong>and</strong><br />
intensities. Then spray with potassium<br />
iodoplatinate reagent <strong>and</strong> repeat the<br />
observations .<br />
4/27/2009 ٨١
Examine the batch of plates making<br />
appropriate comparisons. Spray the plates<br />
within two hours after development <strong>and</strong> read<br />
<strong>and</strong> interpret them as the sprays are applied<br />
<strong>and</strong> again 10 minutes later.<br />
4/27/2009 ٨٢
Methamphetamine (Methedrine ( Methedrine) ) 44(B)<br />
<strong>Amphetamine</strong> (Benzedrine) 54(B)<br />
Solvent System for Test B - Mixture of 200 ml<br />
of methanol <strong>and</strong> 3 ml of concentrated<br />
ammonium hydroxide.<br />
4/27/2009 ٨٣
The ammonia is added to achieve a process known as<br />
ion suppression. suppression.<br />
By converting the drugs to their<br />
free base forms, their polarities are reduced. This is<br />
because the nitrogen atom does not carry a positive<br />
charge in basic solution. The latter reduces the<br />
problem of (TLC) tailing, improves the mass transfer<br />
properties between the stationary <strong>and</strong> mobile phases,<br />
<strong>and</strong> thus improves the chromatographic quality.<br />
4/27/2009 ٨٤
In addition, MDA, MDMA <strong>and</strong> MDEA give rise to<br />
purple, orange/red <strong>and</strong> orange/red products,<br />
respectively. At each of the visualization stages, the<br />
retardation factor (or relative front) (Rf ( Rf) ) values of the<br />
visualized compounds should be calculated by using<br />
the following equation:<br />
Distance moved by the analyte of interest<br />
Rf = -------------------------------------------------------<br />
Distance moved by the solvent front<br />
4/27/2009 ٨٥
The Rf values of the unknowns are compared to those<br />
of the st<strong>and</strong>ards <strong>and</strong> if the data cannot be<br />
discriminated then a suggested match is called.<br />
Although when using this combination of<br />
presumptive tests <strong>and</strong> TLC it is possible to<br />
discriminate within this group of compounds, due to<br />
the extremely large number of amphetamines<br />
available, it is necessary to carry out a confirmatory<br />
con rmatory<br />
analytical technique. The foremost of these, for<br />
amphetamine identification<br />
identi cation, , is gas chromatography–<br />
chromatography<br />
mass spectrometry (GC–MS (GC MS)<br />
4/27/2009 ٨٦
Thin–layer Thin layer Chromatographic Systems<br />
for <strong>Amphetamine</strong><br />
System TA—Rf TA Rf 43; system TB—Rf TB Rf 20;<br />
system TC—Rf TC Rf 09; system TE—Rf TE Rf 43; system<br />
TL—Rf TL Rf 18; system TAE—Rf TAE Rf 12; system<br />
TAF—Rf TAF Rf 75. (Dragendorff spray, positive;<br />
FPN reagent, pink; acidified iodoplatinate<br />
solution, positive; Marquis reagent, brown;<br />
ninhydrin spray, positive; acidified potassium<br />
permanganate solution, positive). positive).<br />
4/27/2009 ٨٧
System TA<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Methanol:strong ammonia<br />
solution (100:1.5).<br />
Reference compounds: compounds:<br />
Atropine Rf 18,<br />
Codeine Rf 33, Chlorprothixene Rf 56,<br />
Diazepam Rf 75.<br />
4/27/2009 ٨٨
Colour test<br />
The Marquis test gives an orange colour for both<br />
amfetamine <strong>and</strong> metamfetamine.<br />
Thin layer chromatography<br />
TA: amfetamine Rf = 0.43, metamfetamine Rf = 0.31.<br />
TB: amfetamine Rf = 0.15, metamfetamine Rf = 0.28.<br />
Visualisation: acidified iodoplatinate solution.<br />
4/27/2009 ٨٩
System TB<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Cyclohexane:toluene<br />
Cyclohexane: toluene:diethylamine<br />
diethylamine (75:15:10).<br />
Reference compounds: compounds:<br />
Codeine Rf 06,<br />
Desipramine Rf 20, Prazepam Rf 36,<br />
Trimipramine Rf 62<br />
4/27/2009 ٩٠
System TC<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Chloroform:methanol (90:10).<br />
Reference compounds: compounds:<br />
Desipramine Rf 11,<br />
Physostigmine Rf 36, Trimipramine Rf 54,<br />
Lidocaine Rf 71.<br />
4/27/2009 ٩١
System TL<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick, dipped in,<br />
or sprayed with, 0.1 M potassium hydroxide in<br />
methanol, <strong>and</strong> dried.<br />
Mobile phase: phase:<br />
Acetone.<br />
Reference compounds: compounds:<br />
Amitriptyline Rf 15,<br />
Procaine Rf 30, Papaverine Rf 47, Cinnarizine<br />
Rf 65.<br />
4/27/2009 ٩٢
System TAE<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick.<br />
Mobile phase: phase:<br />
Methanol.<br />
Reference compounds: compounds:<br />
Codeine Rf 20,<br />
Trimipramine Rf 36, Hydroxyzine Rf 56,<br />
Diazepam Rf 82.<br />
4/27/2009 ٩٣
System TAF<br />
Plates: Plates:<br />
Silica gel G, 250 μm m thick.<br />
Mobile phase: phase:<br />
Methanol:n-butanol Methanol: butanol (60:40) <strong>and</strong><br />
0.1 mol/L NaBr.<br />
Reference compounds: compounds:<br />
Codeine Rf 22,<br />
Diphenhydramine Rf 48, Quinine Rf 65,<br />
Diazepam Rf 85 .<br />
4/27/2009 ٩٤
Location reagents for systems TA, TB<br />
<strong>and</strong> TC<br />
Ninhydrin spray<br />
Spray the plate with the reagent <strong>and</strong> then heat<br />
in an oven at 100° 100 for 5 min. Violet or pink<br />
spots are given by primary amines <strong>and</strong> yellow<br />
colours<br />
Ninhydrin Spray: add 0.5 g of ninhydrin to<br />
10 mL of hydrochloric acid <strong>and</strong> dilute to<br />
100 mL with acetone. Prepare daily.<br />
4/27/2009 ٩٥
FPN reagent<br />
Red or brown-red brown red spots are given by<br />
phenothiazines <strong>and</strong> blue spots by<br />
dibenzazepines. This reagent may be used to<br />
overspray a plate which has been previously<br />
sprayed with ninhydrin spray.<br />
FPN Reagent: mix together 5 mL of ferric<br />
chloride solution, 45 mL of a 20% w/w<br />
solution of perchloric acid, <strong>and</strong> 50 mL of a<br />
50% v/v solution of nitric acid.<br />
4/27/2009 ٩٦
Dragendorff spray<br />
Yellow, orange, red-orange, red orange, or brown-orange brown orange spots<br />
are given by tertiary alkaloids. This reagent may be<br />
used to overspray a plate which has been previously<br />
sprayed with ninhydrin spray <strong>and</strong> FPN spray.<br />
Dragendorff Spray: (a) mix together 2 g of bismuth<br />
subnitrate, subnitrate,<br />
25 mL of acetic acid, <strong>and</strong> 100 mL of<br />
water; (b) dissolve 40 g of potassium iodide in<br />
100 mL of water. Mix together 10 mL of (a), 10 mL<br />
of (b), 20 mL of acetic acid, <strong>and</strong> 100 mL of water.<br />
Prepare every 2 days.<br />
4/27/2009 ٩٧
Acidified iodoplatinate solution<br />
Violet, blue-violet, blue violet, grey-violet, grey violet, or brown-violet brown violet spots<br />
on a pink background are given by tertiary amines<br />
<strong>and</strong> quaternary ammonium compounds. Primary <strong>and</strong><br />
secondary amines give dirtier colours. This solution<br />
may be used to overspray a plate which has<br />
previously been sprayed with ninhydrin spray, FPN<br />
reagent <strong>and</strong> Dragendorff spray.<br />
Iodoplatinate Solution, Acidified: add 5 mL of<br />
hydrochloric acid to 100 mL of iodoplatinate<br />
solution.<br />
4/27/2009 ٩٨
M<strong>and</strong>elin’s M<strong>and</strong>elin s reagent<br />
This reagent is preferably poured onto the<br />
plate because of the danger of spraying<br />
concentrated acid. Many different colours are<br />
given with a variety of drugs<br />
M<strong>and</strong>elin's Reagent: dissolve 0.5 g of<br />
ammonium vanadate in 1.5 mL of water <strong>and</strong><br />
dilute to 100 mL with sulfuric acid. Filter the<br />
solution through glass wool.<br />
4/27/2009 ٩٩
Marquis reagent<br />
This reagent is preferably poured onto the<br />
plate because of the danger of spraying<br />
concentrated acid. Black or violet spots are<br />
given by alkaloids related to morphine. Many<br />
different colours are given with a variety of<br />
drugs<br />
Marquis Reagent: mix 1 mL of<br />
formaldehyde solution with 9 mL of sulfuric<br />
acid. Prepare daily.<br />
4/27/2009 ١٠٠
Acidified potassium permanganate<br />
solution<br />
Yellow-brown Yellow brown spots on a violet background<br />
are given by drugs with unsaturated aliphatic<br />
bonds. bonds<br />
Potassium Permanganate Solution,<br />
Acidified: a 1% solution of potassium<br />
permanganate in 0.25 M sulfuric acid.<br />
4/27/2009 ١٠١
Gas Chromatography.<br />
System GA—amfetamine GA amfetamine RI 1125, amfetamine-TFA amfetamine TFA RI 1095,<br />
amfetamine-PFP amfetamine PFP RI 1330, amfetamine-TMS amfetamine TMS RI 1190,<br />
amfetamine-AC amfetamine AC RI 1501, art (formyl) RI 1100, M (3-OH (3 OH-)-<br />
PFP2 RI 1520, M (3-OH (3 OH-)-TMS2 TMS2 RI 1850, M (3-OH (3 OH-)-AC2 AC2<br />
RI 1930, M (4-OH (4 OH-) ) RI 1480, M (4-OH (4 OH-)-AC2 AC2 RI 1900, M<br />
(3,4–di (3,4 di-OH OH-)-AC3 AC3 RI 2150, M (OH-methoxy<br />
(OH methoxy-) ) RI 1465, M<br />
(OH-methoxy<br />
(OH methoxy-)-AC2 AC2 RI 2065, M (desamino–oxo<br />
(desamino oxo-OH OH-)-AC AC<br />
RI 1520, M (desamino–oxo<br />
(desamino oxo-OH OH-methoxy methoxy-) ) RI 1510, M<br />
(desamino–oxo<br />
(desamino oxo-OH OH-methoxy methoxy-)-AC AC RI 1600, M (desamino– (desamino<br />
oxo–di oxo di-OH OH-)-AC2 AC2 RI 1735; system GB—amfetamine GB amfetamine RI<br />
1150; art (formyl) RI 1142; system GC—RI GC RI 1536; system<br />
GF—RI GF RI 1315; system GAK—retention GAK retention time 4.9 min.<br />
4/27/2009 ١٠٢
Column: DB-5 DB 5 fused silica (30 m × 0.25 mm<br />
i.d., 0.25 μm m film thickness). Column<br />
temperature: 70° 70 for 1 min, ramp to 100° 100 at<br />
30°/min, 30 /min, <strong>and</strong> to 270° 270 at 10°/min. 10 /min. Injector<br />
temperature: 280°. 280 . Carrier gas: helium, flow<br />
rate 0.8 mL/min. MS detection. Retention<br />
time: 6.5 min.<br />
4/27/2009 ١٠٣
High Performance Liquid<br />
Chromatography.<br />
System HA—k HA k 0.9; system HB—k HB k 8.48;<br />
system HC—k HC k 0.98; system HX—RI HX RI 244;<br />
system HAA—retention HAA retention time, 3.7 min; system<br />
HBC—retention HBC retention time 2.1 min; system HBD— HBD<br />
retention time 3.7 min.<br />
4/27/2009 ١٠٤
Column: Chiralcel OD-RH OD RH (150 × 2 mm i.d.,<br />
5 μm) m) at 35°. 35 . Mobile phase: phosphate–citrate<br />
phosphate citrate<br />
buffer (pH 4.0) with sodium<br />
hexafluorophosphate (0.3 M):acetonitrile<br />
(43:57), flow rate 0.1 mL/min. Fluorescence<br />
detection (λex=330 ( ex=330 nm, λem=440 em=440 nm).<br />
Retention time: 24.6 min.<br />
4/27/2009 ١٠٥
Infra–red Infra red Spectrum<br />
Principal peaks at wavenumbers 700, 740, 1495, 1090,<br />
1605, 825 cm−1<br />
cm<br />
4/27/2009 ١٠٦
Definitive De nitive Identification<br />
Identi cation of<br />
<strong>Amphetamine</strong>s<br />
GC–MS GC MS is the preferred method for the identification<br />
identi cation<br />
of amphetamines. The discussion below centres on<br />
the analysis of amphetamine itself, although the same<br />
principles can also be applied to other members of<br />
this class of drug. However, there are a number of<br />
problems associated with the gas chromatographic<br />
analysis of amphetamine. Being highly polar in<br />
nature, this compound is liable to poor<br />
chromatographic behaviour <strong>and</strong> tailing if the<br />
analytical instrument is not scrupulously clean<br />
4/27/2009 ١٠٧
4/27/2009 ١٠٨
4/27/2009 ١٠٩
Furthermore, the highly polar nature of the<br />
amino group results in sorption of<br />
amphetamine to the surfaces of the GC system<br />
components. This, coupled with the often low<br />
concentration of the amphetamine in the<br />
sample, results in the false impression that<br />
there is no amphetamine present in the<br />
specimen under investigation<br />
4/27/2009 ١١٠
In order to alleviate this problem,<br />
derivatization can be employed. One of the<br />
easiest processes, for the analysis of<br />
amphetamine, is to derivatize directly with<br />
carbon disulfide disul de <strong>and</strong> it is this method which<br />
finds nds wide application in the United Kingdom.<br />
For bulk <strong>and</strong> trace samples, this is achieved by<br />
dissolving the material<br />
4/27/2009 ١١١
4/27/2009 ١١٢
The reaction (see equation (2.1)) is a simple,<br />
pre-column pre column derivatization, derivatization,<br />
involving the amino<br />
group of the amphetamine <strong>and</strong> the CS2<br />
.Thisprocess Thisprocess reduces the polarity of the<br />
product, improving its chromatographic<br />
behaviour <strong>and</strong> hence the sensitivity of the<br />
method. In addition, it results in a molecule<br />
which produces characteristic fragments from<br />
the ionization process:<br />
4/27/2009 ١١٣
4/27/2009 ١١٤
Quantification<br />
Quanti cation of <strong>Amphetamine</strong>s<br />
Due to the nature of the compounds being<br />
considered <strong>and</strong> the need for derivatization,<br />
derivatization,<br />
GC–MS GC MS is not considered the best technique<br />
for sample quantification<br />
quanti cation<br />
4/27/2009 ١١٥
There are a number of difficulties dif culties encountered with<br />
quantification<br />
quanti cation after employing derivatization. derivatization.<br />
These<br />
include the fact because derivatization is another<br />
h<strong>and</strong>ling stage in the analytical process, there is<br />
always the risk of sample contamination.<br />
Furthermore, the assumption is made that the<br />
derivatization reactions are ‘complete complete’ <strong>and</strong> that the<br />
corresponding derivatives are stable for the period<br />
between derivative formation <strong>and</strong> analysis. Further<br />
factors are that dilutions need to be extremely<br />
accurate <strong>and</strong> precise to obtain reliable numerical data<br />
<strong>and</strong> that derivatization can potentially lead to<br />
increases in numerical errors for such data .<br />
4/27/2009 ١١٦
The amphetamines (st<strong>and</strong>ards <strong>and</strong> samples) should be<br />
dissolved in methanolic HCl (100 ml of methanol to<br />
which 175 µl l of concentrated HCl has been added). A<br />
range of st<strong>and</strong>ard solutions should be prepared in<br />
order to give a range of concentrations above <strong>and</strong><br />
below that which the street sample is thought to<br />
contain, remembering that the latter may only contain<br />
between 0 <strong>and</strong> 5 wt% amphetamine. If necessary, the<br />
materials (particularly the case samples) should be<br />
sonicated <strong>and</strong>, following this, centrifuged to remove<br />
any solid materials. The supernatant is retained for<br />
subsequent analysis.<br />
4/27/2009 ١١٧
Having collected the data, a calibration curve should be<br />
plotted. Since amphetamine is frequently synthesized in dirty<br />
apparatus in ‘cl<strong>and</strong>estine cl<strong>and</strong>estine’ laboratories, it may not be possible<br />
to determine which salt form of the drug is present. The<br />
st<strong>and</strong>ard is generally supplied as the sulfate form, of the<br />
general formula (amphetamine sulfate). This means that for<br />
every gram of amphetamine sulfate, 73% will be present as the<br />
amphetamine free base. The calibration curve should be<br />
plotted as (UV detector) response against concentration of<br />
amphetamine free base<br />
4/27/2009 ١١٨
Derivatization<br />
4/27/2009 ١١٩
Derivatization results in good chromatographic<br />
behavior <strong>and</strong> provides compounds for which<br />
distinctive mass spectra can be obtained<br />
This method also has the advantage that the most<br />
common adulterant of amphetamine, namely caffeine,<br />
can also be identified identi ed in this system, <strong>and</strong> a suitable<br />
mass spectrum obtained As with all drug<br />
identification<br />
identi cation approaches, if the retention time data<br />
<strong>and</strong> mass spectra of the compounds match, then an<br />
identification<br />
identi cation can be inferred.<br />
4/27/2009 ١٢٠
Mass Spectra<br />
44 91 40 42 65 45 39 43 Amfetamine<br />
44 122 78 121 65 107 91 134<br />
Methoxyamfetamine<br />
44 136 51 135 77 42 78 45<br />
Methylenedioxyamfetamine<br />
44 138 122 137 121 91 78 45<br />
Methylthioamfetamine<br />
4/27/2009 ١٢١