Global Initiative for Chronic Obstructive Lung Disease - GOLD
Global Initiative for Chronic Obstructive Lung Disease - GOLD
Global Initiative for Chronic Obstructive Lung Disease - GOLD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>GOLD</strong>_WR_05 8/18/05 12:56 PM Page 35<br />
remodeling because tissue repair in the airways, as<br />
elsewhere in the body, may involve scar tissue <strong>for</strong>mation.<br />
In any case, this injury-and-repair process results in a<br />
structural remodeling of the airway wall, with increasing<br />
collagen content and scar tissue <strong>for</strong>mation, that narrows<br />
the lumen and produces fixed airways obstruction 87 .<br />
The peripheral airways become the major site of airways<br />
obstruction in COPD, and direct measurements of<br />
peripheral airways resistance 88 show that the structural<br />
changes in the airway wall are the most important cause<br />
of the increase in peripheral airways resistance in COPD.<br />
Inflammatory changes such as airway edema and<br />
mucus hypersecretion also contribute to airway narrowing<br />
in COPD. So does loss of elastic recoil, but fibrosis of<br />
the small airways plays the largest role.<br />
Fibrosis in the peripheral airways, as elsewhere in the<br />
body, is characterized by the accumulation of mesenchymal<br />
cells (fibroblasts and myofibroblasts) and extracellular<br />
connective tissue matrix. Several cell types including<br />
mononuclear phagocytes and epithelial cells may produce<br />
mediators that drive this process. The mediators that<br />
drive the accumulation of these cells and of the matrix<br />
are incompletely defined, but it is likely that several<br />
mediators including TGF-ß, ET-1, Insulin-like growth<br />
factor-1, fibronectin, platelet-derived growth factor<br />
(PDGF), and others are involved 89 .<br />
Figure 4-9. Normal and Emphysematous <strong>Lung</strong>s<br />
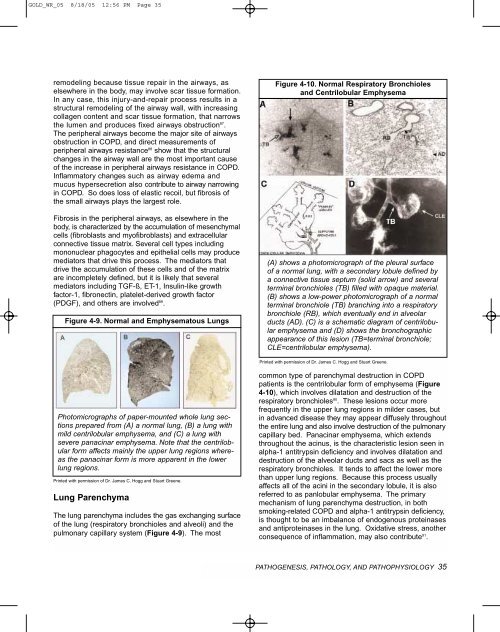
Figure 4-10. Normal Respiratory Bronchioles<br />
and Centrilobular Emphysema<br />
(A) shows a photomicrograph of the pleural surface<br />
of a normal lung, with a secondary lobule defined by<br />
a connective tissue septum (solid arrow) and several<br />
terminal bronchioles (TB) filled with opaque material.<br />
(B) shows a low-power photomicrograph of a normal<br />
terminal bronchiole (TB) branching into a respiratory<br />
bronchiole (RB), which eventually end in alveolar<br />
ducts (AD). (C) is a schematic diagram of centrilobular<br />
emphysema and (D) shows the bronchographic<br />
appearance of this lesion (TB=terminal bronchiole;<br />
CLE=centrilobular emphysema).<br />
Printed with permission of Dr. James C. Hogg and Stuart Greene.<br />
Photomicrographs of paper-mounted whole lung sections<br />
prepared from (A) a normal lung, (B) a lung with<br />
mild centrilobular emphysema, and (C) a lung with<br />
severe panacinar emphysema. Note that the centrilobular<br />
<strong>for</strong>m affects mainly the upper lung regions whereas<br />
the panacinar <strong>for</strong>m is more apparent in the lower<br />
lung regions.<br />
Printed with permission of Dr. James C. Hogg and Stuart Greene.<br />
<strong>Lung</strong> Parenchyma<br />
The lung parenchyma includes the gas exchanging surface<br />
of the lung (respiratory bronchioles and alveoli) and the<br />
pulmonary capillary system (Figure 4-9). The most<br />
common type of parenchymal destruction in COPD<br />
patients is the centrilobular <strong>for</strong>m of emphysema (Figure<br />
4-10), which involves dilatation and destruction of the<br />
respiratory bronchioles 90 . These lesions occur more<br />
frequently in the upper lung regions in milder cases, but<br />
in advanced disease they may appear diffusely throughout<br />
the entire lung and also involve destruction of the pulmonary<br />
capillary bed. Panacinar emphysema, which extends<br />
throughout the acinus, is the characteristic lesion seen in<br />
alpha-1 antitrypsin deficiency and involves dilatation and<br />
destruction of the alveolar ducts and sacs as well as the<br />
respiratory bronchioles. It tends to affect the lower more<br />
than upper lung regions. Because this process usually<br />
affects all of the acini in the secondary lobule, it is also<br />
referred to as panlobular emphysema. The primary<br />
mechanism of lung parenchyma destruction, in both<br />
smoking-related COPD and alpha-1 antitrypsin deficiency,<br />
is thought to be an imbalance of endogenous proteinases<br />
and antiproteinases in the lung. Oxidative stress, another<br />
consequence of inflammation, may also contribute 91 .<br />
PATHOGENESIS, PATHOLOGY, AND PATHOPHYSIOLOGY 35





![Di Bari [NO].pdf - GOLD](https://img.yumpu.com/21544924/1/190x143/di-bari-nopdf-gold.jpg?quality=85)










