You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
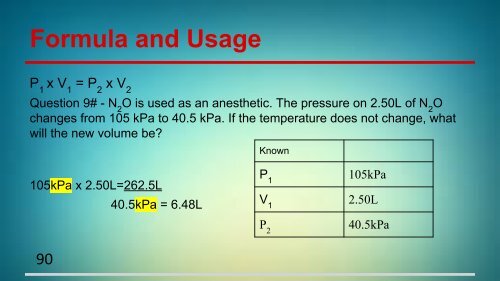
Formula and Usage<br />
P 1<br />
x V 1<br />
= P 2<br />
x V 2<br />
Question 9# - N 2<br />
O is used as an anesthetic. The pressure on 2.50L of N 2<br />
O<br />
changes from 105 kPa to 40.5 kPa. If the temperature does not change, what<br />
will the new volume be?<br />
Known<br />
105kPa x 2.50L=262.5L<br />
40.5kPa = 6.48L<br />
P 1<br />
105kPa<br />
V 1<br />
2.50L<br />
P 2<br />
40.5kPa