Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
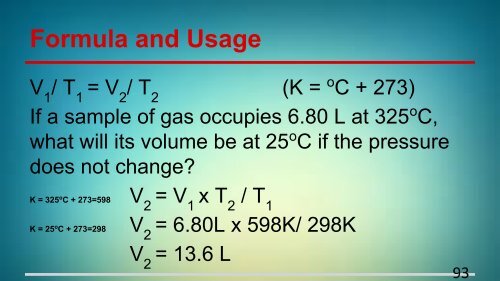
Formula and Usage<br />
V 1<br />
/ T 1<br />
= V 2<br />
/ T 2<br />
(K = o C + 273)<br />
If a sample of gas occupies 6.80 L at 325 o C,<br />
what will its volume be at 25 o C if the pressure<br />
does not change?<br />
K = 325 o C + 273=598 V 2<br />
= V 1<br />
x T 2<br />
/ T 1<br />
K = 25 o C + 273=298 V 2<br />
= 6.80L x 598K/ 298K<br />
V 2<br />
= 13.6 L