You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
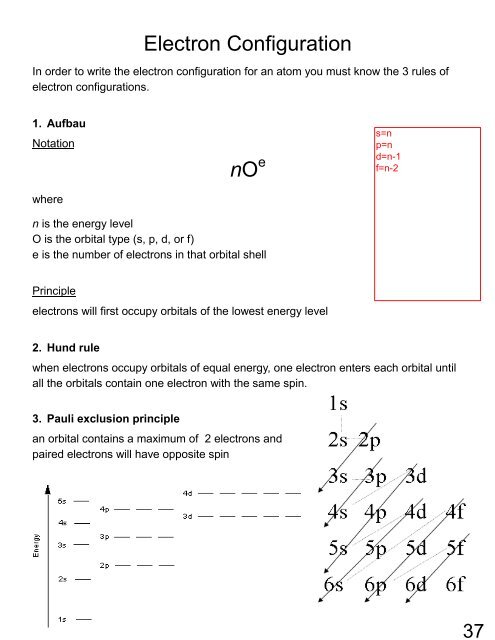
Electron Configuration<br />
In order to write the electron configuration for an atom you must know the 3 rules of<br />
electron configurations.<br />
1. Aufbau<br />
Notation<br />
nO e<br />
where<br />
n is the energy level<br />
O is the orbital type (s, p, d, or f)<br />
e is the number of electrons in that orbital shell<br />
Principle<br />
electrons will first occupy orbitals of the lowest energy level<br />
2. Hund rule<br />
when electrons occupy orbitals of equal energy, one electron enters each orbital until<br />
all the orbitals contain one electron with the same spin.<br />
3. Pauli exclusion principle<br />
an orbital contains a maximum of 2 electrons and<br />
paired electrons will have opposite spin