A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
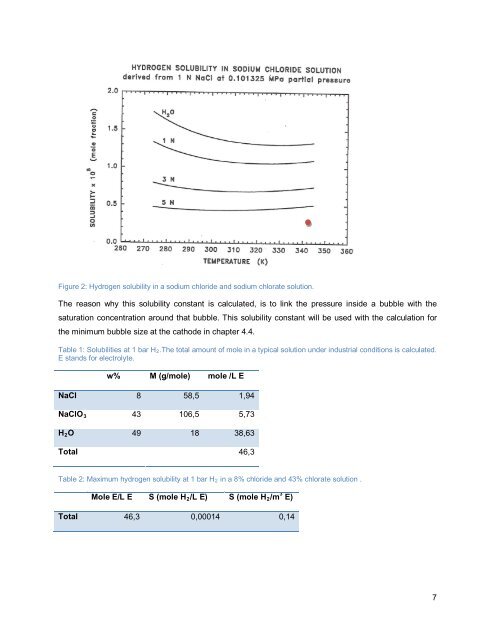
Figure 2: Hydrogen solubility <strong>in</strong> a sodium chloride and sodium <strong>chlorate</strong> solution.<br />
The reason why this solubility constant is calculated, is to l<strong>in</strong>k the pressure <strong>in</strong>side a <strong>bubble</strong> with the<br />
saturation concentration around that <strong>bubble</strong>. This solubility constant will be used with the calculation for<br />
the m<strong>in</strong>imum <strong>bubble</strong> size at the cathode <strong>in</strong> chapter 4.4.<br />
Table 1: Solubilities at 1 bar H 2.The total amount of mole <strong>in</strong> a typical solution under <strong>in</strong>dustrial conditions is calculated.<br />
E stands for electrolyte.<br />
w% M (g/mole) mole /L E<br />
NaCl 8 58,5 1,94<br />
NaClO 3 43 106,5 5,73<br />
H 2 O 49 18 38,63<br />
Total 46,3<br />
Table 2: Maximum hydrogen solubility at 1 bar H 2 <strong>in</strong> a 8% chloride and 43% <strong>chlorate</strong> solution .<br />
Mole E/L E S (mole H 2 /L E) S (mole H 2 /m 3 E)<br />
Total 46,3 0,00014 0,14<br />
7