A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
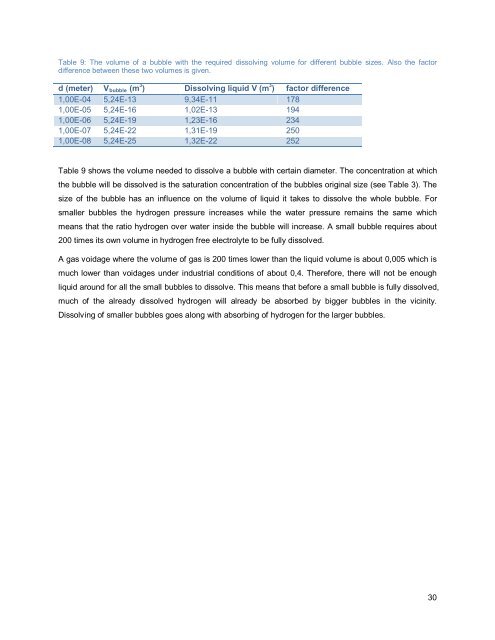
Table 9: The volume of a <strong>bubble</strong> with the required dissolv<strong>in</strong>g volume for different <strong>bubble</strong> sizes. Also the factor<br />
difference between these two volumes is given.<br />
d (meter) V <strong>bubble</strong> (m 3 ) Dissolv<strong>in</strong>g liquid V (m 3 ) factor difference<br />
1,00E-04 5,24E-13 9,34E-11 178<br />
1,00E-05 5,24E-16 1,02E-13 194<br />
1,00E-06 5,24E-19 1,23E-16 234<br />
1,00E-07 5,24E-22 1,31E-19 250<br />
1,00E-08 5,24E-25 1,32E-22 252<br />
Table 9 shows the volume needed to dissolve a <strong>bubble</strong> with certa<strong>in</strong> diameter. The concentration at which<br />
the <strong>bubble</strong> will be dissolved is the saturation concentration of the <strong>bubble</strong>s orig<strong>in</strong>al size (see Table 3). The<br />
size of the <strong>bubble</strong> has an <strong>in</strong>fluence on the volume of liquid it takes to dissolve the whole <strong>bubble</strong>. For<br />
smaller <strong>bubble</strong>s the hydrogen pressure <strong>in</strong>creases while the water pressure rema<strong>in</strong>s the same which<br />
means that the ratio hydrogen over water <strong>in</strong>side the <strong>bubble</strong> will <strong>in</strong>crease. A small <strong>bubble</strong> requires about<br />
200 times its own volume <strong>in</strong> hydrogen free electrolyte to be fully dissolved.<br />
A gas voidage where the volume of gas is 200 times lower than the liquid volume is about 0,005 which is<br />
much lower than voidages under <strong>in</strong>dustrial conditions of about 0,4. Therefore, there will not be enough<br />
liquid around for all the small <strong>bubble</strong>s to dissolve. This means that before a small <strong>bubble</strong> is fully dissolved,<br />
much of the already dissolved hydrogen will already be absorbed by bigger <strong>bubble</strong>s <strong>in</strong> the vic<strong>in</strong>ity.<br />
Dissolv<strong>in</strong>g of smaller <strong>bubble</strong>s goes along with absorb<strong>in</strong>g of hydrogen for the larger <strong>bubble</strong>s.<br />
30