A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4.5 Bubble growth and coalescence<br />
In the <strong>chlorate</strong> process hydrogen <strong>bubble</strong>s are formed. Whether these <strong>bubble</strong>s coalesce or not has great<br />
<strong>in</strong>fluence on the system. It is therefore important to have a better understand<strong>in</strong>g of what coalescence is<br />
and how it can be <strong>in</strong>hibited. If two <strong>bubble</strong>s collide and coalescence occurs, the surface area of the new<br />
<strong>bubble</strong> will be smaller than that of the two small <strong>bubble</strong>s together. The change of surface energy due to<br />
coalescence is given as<br />
∆E = σ gas−liquid ∆A (4.31)<br />
S<strong>in</strong>ce the surface area of the newly formed <strong>bubble</strong> will be smaller than before, the energy change will<br />
always be negative. The process of two coalesc<strong>in</strong>g <strong>bubble</strong>s will result <strong>in</strong> a lower state of energy. This<br />
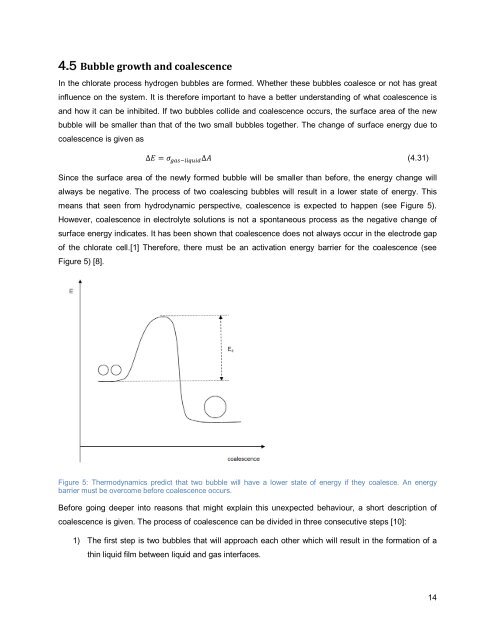
means that seen from hydrodynamic perspective, coalescence is expected to happen (see Figure 5).<br />
However, coalescence <strong>in</strong> electrolyte solutions is not a spontaneous process as the negative change of<br />
surface energy <strong>in</strong>dicates. It has been shown that coalescence does not always occur <strong>in</strong> the electrode gap<br />
of the <strong>chlorate</strong> cell.[1] Therefore, there must be an activation energy barrier for the coalescence (see<br />
Figure 5) [8].<br />
Figure 5: Thermodynamics predict that two <strong>bubble</strong> will have a lower state of energy if they coalesce. An energy<br />
barrier must be overcome before coalescence occurs.<br />
Before go<strong>in</strong>g deeper <strong>in</strong>to reasons that might expla<strong>in</strong> this unexpected behaviour, a short description of<br />
coalescence is given. The process of coalescence can be divided <strong>in</strong> three consecutive steps [10]:<br />
1) The first step is two <strong>bubble</strong>s that will approach each other which will result <strong>in</strong> the formation of a<br />
th<strong>in</strong> liquid film between liquid and gas <strong>in</strong>terfaces.<br />
14