A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
A bubble curtain model applied in chlorate electrolysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.3 Results and discussion<br />
Table 10 and Table 11 present the results for two and three liter electrolyte. This gave levels of 32 and<br />
respectively 44 mL above the start<strong>in</strong>g level where the voidage was zero. In both cases two electrodes are<br />
used, which means there was one electrode gap of 15 cm 2 .<br />
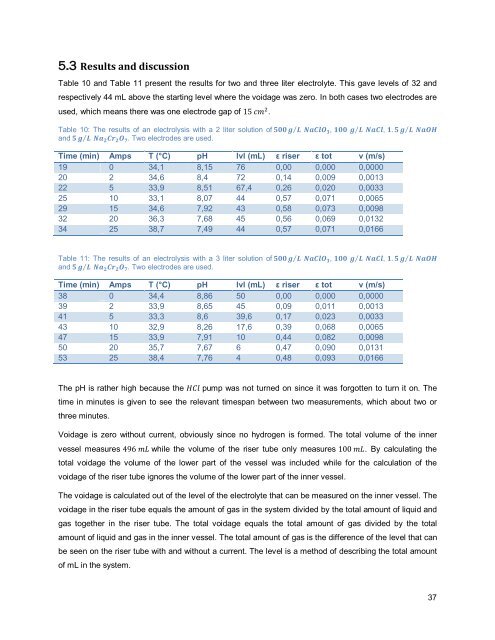
Table 10: The results of an <strong>electrolysis</strong> with a 2 liter solution of 500 g⁄ L NaClO 3 , 100 g⁄ L NaCl, 1. 5 g⁄ L NaOH<br />
and 5 g⁄ L Na 2 Cr 2 O 7 . Two electrodes are used.<br />
Time (m<strong>in</strong>) Amps T (°C) pH lvl (mL) ε riser ε tot v (m/s)<br />
19 0 34,1 8,15 76 0,00 0,000 0,0000<br />
20 2 34,6 8,4 72 0,14 0,009 0,0013<br />
22 5 33,9 8,51 67,4 0,26 0,020 0,0033<br />
25 10 33,1 8,07 44 0,57 0,071 0,0065<br />
29 15 34,6 7,92 43 0,58 0,073 0,0098<br />
32 20 36,3 7,68 45 0,56 0,069 0,0132<br />
34 25 38,7 7,49 44 0,57 0,071 0,0166<br />
Table 11: The results of an <strong>electrolysis</strong> with a 3 liter solution of 500 g⁄ L NaClO 3 , 100 g⁄ L NaCl, 1. 5 g⁄ L NaOH<br />
and 5 g⁄ L Na 2 Cr 2 O 7 . Two electrodes are used.<br />
Time (m<strong>in</strong>) Amps T (°C) pH lvl (mL) ε riser ε tot v (m/s)<br />
38 0 34,4 8,86 50 0,00 0,000 0,0000<br />
39 2 33,9 8,65 45 0,09 0,011 0,0013<br />
41 5 33,3 8,6 39,6 0,17 0,023 0,0033<br />
43 10 32,9 8,26 17,6 0,39 0,068 0,0065<br />
47 15 33,9 7,91 10 0,44 0,082 0,0098<br />
50 20 35,7 7,67 6 0,47 0,090 0,0131<br />
53 25 38,4 7,76 4 0,48 0,093 0,0166<br />
The pH is rather high because the HCl pump was not turned on s<strong>in</strong>ce it was forgotten to turn it on. The<br />
time <strong>in</strong> m<strong>in</strong>utes is given to see the relevant timespan between two measurements, which about two or<br />
three m<strong>in</strong>utes.<br />
Voidage is zero without current, obviously s<strong>in</strong>ce no hydrogen is formed. The total volume of the <strong>in</strong>ner<br />
vessel measures 496 mL while the volume of the riser tube only measures 100 mL. By calculat<strong>in</strong>g the<br />
total voidage the volume of the lower part of the vessel was <strong>in</strong>cluded while for the calculation of the<br />
voidage of the riser tube ignores the volume of the lower part of the <strong>in</strong>ner vessel.<br />
The voidage is calculated out of the level of the electrolyte that can be measured on the <strong>in</strong>ner vessel. The<br />
voidage <strong>in</strong> the riser tube equals the amount of gas <strong>in</strong> the system divided by the total amount of liquid and<br />
gas together <strong>in</strong> the riser tube. The total voidage equals the total amount of gas divided by the total<br />
amount of liquid and gas <strong>in</strong> the <strong>in</strong>ner vessel. The total amount of gas is the difference of the level that can<br />
be seen on the riser tube with and without a current. The level is a method of describ<strong>in</strong>g the total amount<br />
of mL <strong>in</strong> the system.<br />
37