Kyanite, Sillimanite, and Andalusite Deposits of the Southeastern ...
Kyanite, Sillimanite, and Andalusite Deposits of the Southeastern ...
Kyanite, Sillimanite, and Andalusite Deposits of the Southeastern ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ORIGIN 21<br />
<strong>of</strong> temperature <strong>and</strong> pressure during complex<br />
<strong>the</strong>rmal metamorphism superimposed on a regional<br />
metamorphism.<br />
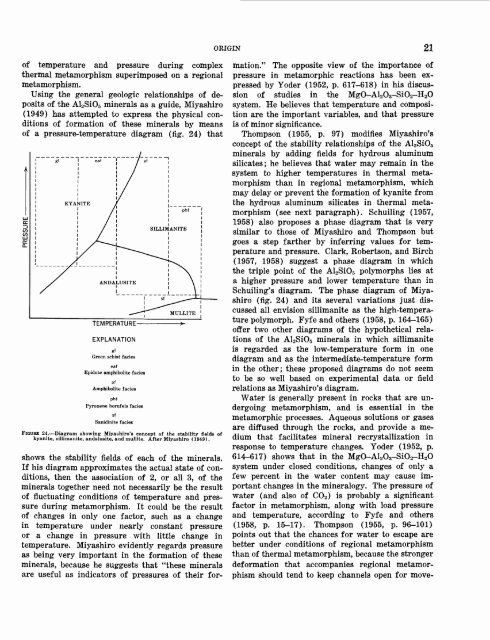
Using <strong>the</strong> general geologic relationships <strong>of</strong> deposits<br />
<strong>of</strong> <strong>the</strong> Al2SiO5 minerals as a guide, Miyashiro<br />
(1949) has attempted to express <strong>the</strong> physical conditions<br />
<strong>of</strong> formation <strong>of</strong> <strong>the</strong>se minerals by means<br />
<strong>of</strong> a pressure-temperature diagram (fig. 24) that<br />
TEMPERATURE<br />
EXPLANATION<br />
gf<br />
Green schist facies<br />
eaf<br />
Epidote amphibolite facies<br />
af<br />
Amphibolite facies<br />
phf<br />
Pyroxene hornfels facies<br />
sf<br />
Sanidinite facies<br />
FIGURE 24. Diagram showing Miyashiro's concept <strong>of</strong> <strong>the</strong> stability fields <strong>of</strong><br />
kyanite, sillimanite, <strong>and</strong>alusite, <strong>and</strong> mullite. After Miyashiro (1949).<br />
shows <strong>the</strong> stability fields <strong>of</strong> each <strong>of</strong> <strong>the</strong> minerals.<br />
If his diagram approximates <strong>the</strong> actual state <strong>of</strong> conditions,<br />
<strong>the</strong>n <strong>the</strong> association <strong>of</strong> 2, or all 3, <strong>of</strong> <strong>the</strong><br />
minerals toge<strong>the</strong>r need not necessarily be <strong>the</strong> result<br />
<strong>of</strong> fluctuating conditions <strong>of</strong> temperature <strong>and</strong> pressure<br />
during metamorphism. It could be <strong>the</strong> result<br />
<strong>of</strong> changes in only one factor, such as a change<br />
in temperature under nearly constant pressure<br />
or a change in pressure with little change in<br />
temperature. Miyashiro evidently regards pressure<br />
as being very important in <strong>the</strong> formation <strong>of</strong> <strong>the</strong>se<br />
minerals, because he suggests that "<strong>the</strong>se minerals<br />
are useful as indicators <strong>of</strong> pressures <strong>of</strong> <strong>the</strong>ir formation."<br />
The opposite view <strong>of</strong> <strong>the</strong> importance <strong>of</strong><br />
pressure in metamorphic reactions has been expressed<br />
by Yoder (1952, p. 617-618) in his discussion<br />
<strong>of</strong> studies in <strong>the</strong> MgO-Al2O3-SiO2-H20<br />
system. He believes that temperature <strong>and</strong> composition<br />
are <strong>the</strong> important variables, <strong>and</strong> that pressure<br />
is <strong>of</strong> minor significance.<br />
Thompson (1955, p. 97) modifies Miyashiro's<br />
concept <strong>of</strong> <strong>the</strong> stability relationships <strong>of</strong> <strong>the</strong> Al2SiO5<br />
minerals by adding fields for hydrous aluminum<br />
silicates; he believes that water may remain in <strong>the</strong><br />
system to higher temperatures in <strong>the</strong>rmal metamorphism<br />
than in regional metamorphism, which<br />
may delay or prevent <strong>the</strong> formation <strong>of</strong> kyanite from<br />
<strong>the</strong> hydrous aluminum silicates in <strong>the</strong>rmal metamorphism<br />
(see next paragraph). Schuiling (1957,<br />
1958) also proposes a phase diagram that is very<br />
similar to those <strong>of</strong> Miyashiro <strong>and</strong> Thompson but<br />
goes a step far<strong>the</strong>r by inferring values for temperature<br />
<strong>and</strong> pressure. Clark, Robertson, <strong>and</strong> Birch<br />
(1957, 1958) suggest a phase diagram in which<br />
<strong>the</strong> triple point <strong>of</strong> <strong>the</strong> Al2SiO 5 polymorphs lies at<br />
a higher pressure <strong>and</strong> lower temperature than in<br />
Schuiling's diagram. The phase diagram <strong>of</strong> Miyashiro<br />
(fig. 24) <strong>and</strong> its several variations just discussed<br />
all envision sillimanite as <strong>the</strong> high-temperature<br />
polymorph. Fyfe <strong>and</strong> o<strong>the</strong>rs (1958, p. 164-165)<br />
<strong>of</strong>fer two o<strong>the</strong>r diagrams <strong>of</strong> <strong>the</strong> hypo<strong>the</strong>tical relations<br />
<strong>of</strong> <strong>the</strong> AloSiO5 minerals in which sillimanite<br />
is regarded as <strong>the</strong> low-temperature form in one<br />
diagram <strong>and</strong> as <strong>the</strong> intermediate-temperature form<br />
in <strong>the</strong> o<strong>the</strong>r; <strong>the</strong>se proposed diagrams do not seem<br />
to be so well based on experimental data or field<br />
relations as Miyashiro's diagram.<br />
Water is generally present in rocks that are undergoing<br />
metamorphism, <strong>and</strong> is essential in <strong>the</strong><br />
metamorphic processes. Aqueous solutions or gases<br />
are diffused through <strong>the</strong> rocks, <strong>and</strong> provide a medium<br />
that facilitates mineral recrystallization in<br />
response to temperature changes. Yoder (1952, p.<br />
614-617) shows that in <strong>the</strong> MgO-Al2O3-SiO2-H2O<br />
system under closed conditions, changes <strong>of</strong> only a<br />
few percent in <strong>the</strong> water content may cause important<br />
changes in <strong>the</strong> mineralogy. The pressure <strong>of</strong><br />
water (<strong>and</strong> also <strong>of</strong> C0 2 ) is probably a significant<br />
factor in metamorphism, along with load pressure<br />
<strong>and</strong> temperature, according to Fyfe <strong>and</strong> o<strong>the</strong>rs<br />
(1958, p. 15-17). Thompson (1955, p. 96-101)<br />
points out that <strong>the</strong> chances for water to escape are<br />
better under conditions <strong>of</strong> regional metamorphism<br />
than <strong>of</strong> <strong>the</strong>rmal metamorphism, because <strong>the</strong> stronger<br />
deformation that accompanies regional metamorphism<br />
should tend to keep channels open for move-