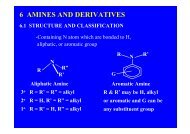
Organometallic compounds
Organometallic compounds
Organometallic compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Organometallic</strong> Compounds<br />
By<br />
Thara Manangan<br />
http://www.ic.kmutnb.ac.th/tmg/411208/
Reactions of Alkyl Halides with Reducing Metals<br />
Victor Grignard<br />
French chemist, 1871-1935<br />
Li, Na, K, … ,Mg and Ca and Zn are good reducing agents<br />
Halide reactivity increases in the order: Cl < Br < I<br />
R 3 C‐X + 2Li ——> R 3 C‐Li + LiX An Alkyl Lithium Reagent<br />
R 3 C‐X + Mg ——> R 3 C‐MgX<br />
A Grignard Reagent
Some Reactions of Organolithium and Grignard Reagents<br />
R‐X + Zn –––> R‐Zn‐X An Alkyl Zinc Reagent 1850 E. Frankland<br />
R‐X + Mg –––> R‐Mg‐X A Grignard Reagent 1900 V. Grignard<br />
R‐X + 2Li –––> R‐Li + LiX<br />
An Alkyl Lithium Reagent 1917 W. Schlenk (1930 K. Ziegler)
Some Reactions of Organolithium and Grignard Reagents<br />
Metal Exchange<br />
2 CH 3 Li + CuI ——> (CH 3 ) 2 CuLi + LiI A Gilman Reagent<br />
(C 3 H 7 ) 2 CuLi + C 6 H 5 I ——> C 6 H 5 ‐C 3 H 7 + LiI + C 3 H 7 Cu A Coupling Reaction
Metal Exchange Reactions<br />
Alternative methods of preparing a wide variety of organometallic <strong>compounds</strong><br />
generally involve an exchange reaction in which a given metal is replaced by a new<br />
metal, which may include B, Al, Ti, V, Fe, Ni, Cu, Mo, Ru, Pd, Sn, Pt, Hg & Pb.
Metal Exchange Reactions
Metal Exchange Reactions<br />
Typical Directed Electrophilic Substitution
Directed ortho‐Metalation (DoM)
Directing Metalation Group (DMG)
Directed ortho‐Metalation (DoM)
Application of Directed ortho‐Metalation
Reactions of <strong>Organometallic</strong> Compounds
Reactions of <strong>Organometallic</strong> Compounds
Reactions of <strong>Organometallic</strong> Compounds
Reactions of <strong>Organometallic</strong> Compounds
Reactions of <strong>Organometallic</strong> Compounds<br />
<strong>Organometallic</strong> Reagents from Geminal Dihalides
<strong>Organometallic</strong> Reagents from Geminal Dihalides
Functionalized <strong>Organometallic</strong> Reagents
Functionalized <strong>Organometallic</strong> Reagents
Applications of Transition Metals to Organic Chemistry
Some Typical Reactions of Transition Metal Complexes
Examples of Oxidative Addition and Reductive Elimination
Examples of Insertion and Elimination Reactions
‐Bonding in Transition Metal Complexes<br />
Hydrogenation Using Transition Metal Catalysts
Wilkinson’s Rhodium Catalysts<br />
Keck’s Allylation
The 2010 Nobel Prize in chemistry: Heck, Negishi and Suzuki<br />
Carbon‐Carbon Bond Formation
Carbon‐Carbon Bond Formation
Carbon‐Carbon Bond Formation
Carbon‐Carbon Bond Formation
Carbon‐Carbon Bond Formation
Carbon‐Carbon Bond Formation
Alkylidene Complexes<br />
Schrock alkylidenes and Fischer carbenes
Alkylidene Complexes<br />
Schrock alkylidenes and Fischer carbenes
Alkylidene Complexes<br />
Schrock alkylidenes and Fischer carbenes
Fisher Carbene Reactions
Fisher Carbene Reactions
Fisher Carbene Reactions
Fisher Carbene Reactions
Alkylidene Reactions
Alkylidene Reactions
Alkylidene Reactions
Metathesis Catalysts<br />
Ring Opening Metathesis Polymerization (ROPM)<br />
Y. Chauvin, R. Grubbs and R. Schrock won the 2005 Nobel Prize in chemistry
Ring Closing Metathesis (RCM)
Ring Closing Metathesis (RCM)