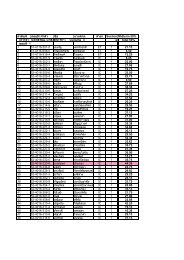
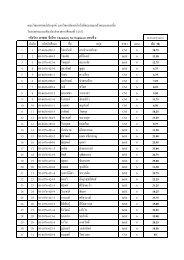
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Alpha rays ():<br />
Beta rays ():<br />
Gamma rays (<br />
•Ernest Rutherford<br />
•1871-1937<br />
2
0.01 mm 1 mm 100 mm<br />
Pieces of lead<br />
3
(Z) = <br />
(A) = + <br />
= (Z) + <br />
Mass Number<br />
Atomic Number<br />
A<br />
Z<br />
X<br />
Element Symbol<br />
4
Particle Symbol <strong>Nuclear</strong><br />
symbol<br />
Proton p +<br />
1<br />
H 1 p<br />
1 1<br />
Neutron n 0<br />
1<br />
n<br />
0<br />
Electron e -<br />
0<br />
e<br />
1<br />
Alpha<br />
<br />
4<br />
2<br />
Beta <br />
0<br />
1 <br />
Positron 0<br />
4 2He<br />
0 1e<br />
0 1e<br />
1 <br />
5
74<br />
33<br />
As 60<br />
27<br />
Co<br />
226<br />
88 Ra 15<br />
32 P<br />
59 Fe<br />
24 Na<br />
99 Tc<br />
3 H<br />
Strontium-90 Iodine-131 Carbon-6<br />
6
ISOTOPES<br />
<br />
1<br />
1 H 2 1 H 3 1 H<br />
12<br />
6 C 13 6 C 14 6 C<br />
235<br />
92 U 238<br />
92 U<br />
7
MASS SPECTROSCOPY<br />
<br />
m<br />
<br />
B<br />
2<br />
E<br />
qr<br />
8
Balancing <strong>Nuclear</strong> Equations<br />
1. Conserve mass number (A).<br />
<br />
235 U<br />
92<br />
+ 138<br />
0<br />
55<br />
Cs<br />
1<br />
n<br />
+ 96 37<br />
Rb + 2 1<br />
n 0<br />
235 + 1 = 138 + 96 + 2x1<br />
2. Conserve atomic number (Z) or nuclear charge.<br />
<br />
235 U<br />
92<br />
+ 138<br />
0<br />
Cs<br />
55<br />
1<br />
n<br />
+ 96 37<br />
Rb + 2 1<br />
n 0<br />
92 + 0 = 55 + 37 + 2x0<br />
10
212<br />
Po alpha 212 Po.<br />
alpha particle -<br />
4<br />
He<br />
2<br />
or<br />
4<br />
2<br />
212<br />
Po 4<br />
He + A X<br />
84 2 Z<br />
212 = 4 + A A = 208<br />
84 = 2 + Z Z = 82<br />
212<br />
Po 4<br />
He + 208 Pb<br />
84 2 82<br />
11
12
13
222<br />
88<br />
Ra<br />
<br />
4<br />
2<br />
He<br />
<br />
218<br />
86<br />
Rn<br />
14
15
16
17
18
n/p<br />
<br />
beta decay<br />
X<br />
‣ (stable isotope) <br />
<br />
(Radioisotope) <br />
(Radioisotope)<br />
Y<br />
‣ p < 20 n/p = 1 <br />
‣ 21 < p < 83 n/p >1<br />
<br />
‣ p > 83 <br />
n/p<br />
<br />
positron decay or electron capture<br />
19
-FILM BAGGES<br />
<br />
20
- GEIGER-MULLER COUNTER<br />
-<br />
<br />
<br />
21
-CLOUD CHAMBER<br />
(Cloud chamber)<br />
<br />
cloud chamber photograph of pair production<br />
22
(NATURAL RADIOACTIVITY-HALF-LIFE)<br />
23
HALF-LIVES OF VARIOUS NUCLIDES<br />
Nuclide Half-life Type of decay<br />
Th-232 1.4 x 10 10 yr Alpha<br />
U-238 4.5 x 10 9 yr Alpha<br />
C-14 5730 yr Beta<br />
Rn-220 55.6 sec Alpha<br />
Th-219 1.05 x 10 –6 sec Alpha<br />
24
25
(OBJECT DATING)<br />
14<br />
N + 1 n<br />
14<br />
C + 1 H<br />
7 0 6 1<br />
14<br />
C<br />
14<br />
N + 0 <br />
6 7 -1<br />
t ½ = 5730 years<br />
26
RADIOCARBON DATING<br />
% C-14 (compared to<br />
living organism)<br />
Object’s age (in years)<br />
100% 0<br />
90% 870<br />
80% 1850<br />
60% 4220<br />
50% 5730<br />
40% 7580<br />
25% 11,500<br />
10% 19,000<br />
5% 24,800<br />
1% 38,100 27
(OBJECT DATING)<br />
Uranium-238 Dating<br />
238<br />
U<br />
206<br />
Pb + 8 4 + 6 0 <br />
92 82 2 -1<br />
t ½ = 4.51 x 10 9 years<br />
-<br />
28
- (NUCLEAR FISSION)<br />
+ energy!!<br />
?<br />
29
235<br />
U + 1 n<br />
90<br />
Sr + 143 Xe + 3 1 n + Energy<br />
92 0 38 54 0<br />
30
+<br />
31
o U-235<br />
o Cd B<br />
o Moderator <br />
H 2 O D 2 O<br />
o<br />
o <br />
32
- (NUCLEAR FUSION)<br />
<br />
<br />
<br />
<br />
33
(RADIOISOTOPES IN MEDICINE)<br />
• (tracer)<br />
•<br />
24<br />
Na, t ½ = 14.8 hr, b emitter, blood-flow tracer<br />
•<br />
131<br />
I, t ½ = 14.8 hr, b emitter, thyroid gland activity<br />
•<br />
123<br />
I, t ½ = 13.3 hr, g-ray emitter, brain imaging<br />
•<br />
18<br />
F, t ½ = 1.8 hr, b + emitter, positron emission tomography (PET)<br />
•<br />
99m<br />
Tc, t ½ = 6 hr, g-ray emitter, imaging agent<br />
Brain images with 123 I-<br />
labeled compound<br />
34
- TREATMENT RADIOTHERAPY<br />
<br />
Co-60<br />
35
-<br />
36
-<br />
37
-SMOKE DETECTOR<br />
38
BIOLOGICAL EFFECTS OF RADIATION<br />
The amount of danger to humans of<br />
radiation is measured in the unit rems.<br />
Dose (rems)<br />
Probable outcome<br />
20–100<br />
100–400<br />
500+ Death<br />
Decreased white blood cell count;<br />
possible increased cancer risk<br />
Radiation sickness;<br />
increased cancer risk<br />
39
Biological Effects of Radiation<br />
Radiation absorbed dose (rad)<br />
1 rad = 1 x 10 -5 J/g of material<br />
Roentgen equivalent for man (rem)<br />
1 rem = 1 rad x Q Quality Factor<br />
-ray = 1<br />
= 1<br />
= 20<br />
40