Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chapter</strong> <strong>2.</strong> <strong>POLAR</strong> <strong>ADDITION</strong> <strong>AND</strong> <strong>ELIMINATION</strong> <strong>REACTIONS</strong><br />
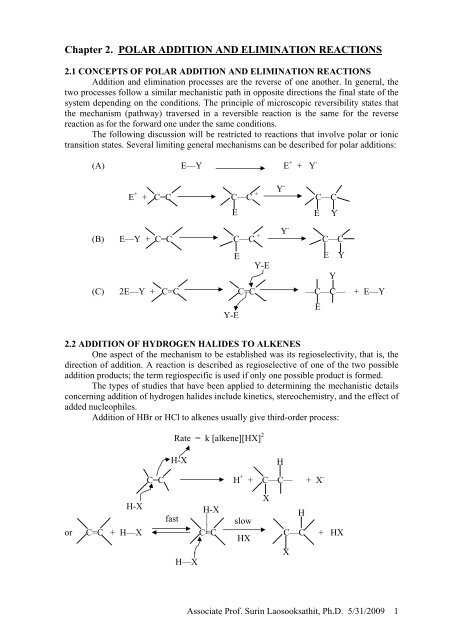
<strong>2.</strong>1 CONCEPTS OF <strong>POLAR</strong> <strong>ADDITION</strong> <strong>AND</strong> <strong>ELIMINATION</strong> <strong>REACTIONS</strong><br />
Addition and elimination processes are the reverse of one another. In general, the<br />
two processes follow a similar mechanistic path in opposite directions the final state of the<br />
system depending on the conditions. The principle of microscopic reversibility states that<br />
the mechanism (pathway) traversed in a reversible reaction is the same for the reverse<br />
reaction as for the forward one under the same conditions.<br />
The following discussion will be restricted to reactions that involve polar or ionic<br />
transition states. Several limiting general mechanisms can be described for polar additions:<br />
(A) E—Y E + + Y -<br />
E + + C=C C—C + C—C<br />
(B) E—Y + C=C C—C + C—C<br />
E<br />
E<br />
(C) 2E—Y + C=C C=C —C—C— + E—Y<br />
Y-E<br />
Y-E<br />
Y -<br />
Y -<br />
E<br />
E<br />
Y<br />
E Y<br />
Y<br />
<strong>2.</strong>2 <strong>ADDITION</strong> OF HYDROGEN HALIDES TO ALKENES<br />
One aspect of the mechanism to be established was its regioselectivity, that is, the<br />
direction of addition. A reaction is described as regioselective of one of the two possible<br />
addition products; the term regiospecific is used if only one possible product is formed.<br />
The types of studies that have been applied to determining the mechanistic details<br />
concerning addition of hydrogen halides include kinetics, stereochemistry, and the effect of<br />
added nucleophiles.<br />
Addition of HBr or HCl to alkenes usually give third-order process:<br />
Rate = k [alkene][HX] 2<br />
H-X<br />
H<br />
C=C H + + C—C— + X -<br />
H-X<br />
X<br />
H-X<br />
fast<br />
slow<br />
H<br />
or C=C + H—X C=C<br />
HX<br />
C—C + HX<br />
X<br />
H—X<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 1
The stereochemistry of addition of HX to unconjugated alkenes is predominantly<br />
anti. Temperature and solvent can modify the stereochemistry, i.e. syn at lower<br />
temperature.<br />
A significant variation in the stereochemistry takes place when the double bond is<br />
conjugated with a group than can stabilize a carbocation intermediate.<br />
+ X- syn<br />
X<br />
ArCH=CHR + HX ArCH—CHR ARCH-CH 2 R<br />
H<br />
Rate is second order<br />
Ion pair<br />
Show salt effect<br />
+ HCl the added Cl - will increase the rate<br />
+ HBr the added Br - will increase the rate<br />
Skeletal rearrangements have been observed.<br />
HCl<br />
CH 3 NO 2<br />
+<br />
40% Cl<br />
Cl<br />
60%<br />
HCl<br />
CH 3 NO 2<br />
Cl<br />
+<br />
Cl<br />
17 % 83%<br />
D<br />
DBr<br />
D<br />
Br<br />
+<br />
Br<br />
DCl<br />
57%<br />
D<br />
Cl<br />
D<br />
+ +<br />
Cl<br />
Cl<br />
41%<br />
2%<br />
D<br />
<strong>2.</strong>3 ACID-CATALYZED HYDRATION OF ALKENES<br />
H +<br />
R—CH=CH 2 R—CH—CH 3<br />
H 2 O<br />
OH<br />
Several points have to be considered :<br />
-Is the protonation step reversible?<br />
-Is there a discrete carbocation?<br />
-Does the nucleophile become involved before carbocation formation is complete?<br />
-Is there any rearrangment taking place?<br />
For simple alkene, it is not easy to study mechanistically.There are two reasons for<br />
the difficulty:<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 2
(1) Simple alkenes and water are not mutually soluble, except in very concentrated<br />
acids.<br />
(2) The spectral features of simple alkenes do not allow for very convenient<br />
methods of following reaction rates, absorption in UV below 200 nm.<br />
For this reason, most studies have been done with conjugated alkenes<br />
particularly styrenes.<br />
slow<br />
H + + -H +<br />
PhCH=CD 2 PhCHCD 2 H PhCH=CHD<br />
H 2 O , fast<br />
PhCH-CD 2 H<br />
OH<br />
This mechanism is correspond well with, i.e. rate to reaction increases with<br />
electron-releasing substituents. A substantial solvent isotope effect k H2O /k D2O = 2-4 is<br />
observed.<br />
<strong>2.</strong>3 <strong>ADDITION</strong> OF HALOGENS<br />
C=C + X 2 —C—C—<br />
X X<br />
-Is the reaction concerted ?<br />
-Is there a discrete positively charged intermediate, carbocation or a cyclic<br />
halonium ion?<br />
For brominations, anti addition is preferred for simple alkenes, for conjugated<br />
alkenes with aryl groups, the extent of syn addition becomes dominant pathway.<br />
Chlorination is not so stereoselective as bromination, but tends to follow the same pattern<br />
in a lesser extend.<br />
Br - Br + Br - Br<br />
Br<br />
syn<br />
Br<br />
C C C—C C C<br />
+<br />
anti<br />
The existence of halonium ion<br />
CH 3 CHCH 2 Br<br />
SbF 5 /SO 2<br />
CH 3 CH—CH 2<br />
-60 o C<br />
-<br />
SbF 6<br />
F<br />
Br<br />
+<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 3
∆∆H #<br />
from steric repulsions<br />
in transition state<br />
cis<br />
trans<br />
Bromination transition state<br />
resembles a three-membered ring<br />
∆∆H<br />
from steric repulsions in<br />
ground state<br />
In general, the difference in enthalpy of the transition states for bromination was<br />
greater than the enthalpy difference of the isomeric alkenes, This finding is consistent with<br />
a cyclic somewhat closer together than in the alkene.<br />
The kinetics of brominations are often complex, with at least three term making<br />
contributions under given conditions:<br />
Rate = k 1 [alkene][Br 2 ] + k 2 [alkene][Br 2 ] 2 + k 3 [alkene][Br 2 ][Br - ]<br />
Br 2<br />
+Br<br />
Br 2<br />
Br 2<br />
Br<br />
Br -<br />
Br<br />
C=C C=C C — C C—C—<br />
Br 2<br />
Relative reactivity of alkenes toward halogenation<br />
Alkene Chlorination Bromination<br />
1.00 1.00<br />
1.15 0.12<br />
63 27<br />
50 17.5<br />
58 57<br />
11,000 13,700<br />
The Hammett correlation for bromination of styrenes is best with σ + substituent<br />
constants and, gives ρ = -4.8. The rates of reactions increase with solvent polarity and<br />
with additional substitution of electron-releasing alkyl groups at the double bond. All of<br />
these features are in accord with an electrophilic mechanism.<br />
Chlorination generally exhibits second-order kinetics and usually leads to expulsion<br />
of a proton from the chloronium ion intermediate.<br />
R<br />
R 2 C<br />
Cl +<br />
R Cl<br />
R<br />
R<br />
C C C C + H +<br />
R<br />
R 2 C<br />
R<br />
H<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 4
<strong>2.</strong>4 THE E2, E1, <strong>AND</strong> E1cb MECHANISMS<br />
An elimination reaction -the expulsion of a small molecule from an organic<br />
substrate- can be classified according to the relative placement of the carbon atoms from<br />
which elimination occurs:<br />
H<br />
..<br />
—C—X C + HX α-elim.<br />
H<br />
X<br />
—C —C C=C + HX β-elim.<br />
H<br />
X<br />
—C—C—C— C C + HX γ-elim.<br />
The β-eliminations can be further subdivided by closer examination of the mechanisms<br />
involved.<br />
E1<br />
B:<br />
—C—C + — C=C + B:H +<br />
slow<br />
H<br />
fast<br />
X<br />
X<br />
E2<br />
—C—C— —C — C— BH + + C=C + X -<br />
B:<br />
H<br />
H<br />
B<br />
E1cb<br />
B:<br />
X -<br />
B:H + + C C C=C + X -<br />
Variable-transition-state theory was proposed to cope with the intermediate<br />
mechanisms.<br />
Increasing C-H breaking in the transition state<br />
C<br />
X<br />
B-H δ+ δ-<br />
B-H δ+ δ-<br />
B—H δ+<br />
B H δ+<br />
H<br />
E1cb<br />
X<br />
X<br />
E1cb-like<br />
X<br />
Synchronous E2<br />
E1-like<br />
δ+<br />
X δ-<br />
E1<br />
δ+<br />
X δ-<br />
The most important structural features to be considered concerning the mechanistic<br />
types are:<br />
(1) The nature of the leaving group<br />
(2) The nature of the base<br />
(3) Steric factors in the substrate<br />
(4) Solvent effects<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 5
Br<br />
KOBu -t<br />
85%<br />
Br<br />
KOBu -t<br />
30-40%<br />
Br<br />
CH 3<br />
Cl<br />
3COK<br />
+<br />
75% 25%<br />
Ph<br />
Br<br />
Br<br />
O<br />
AcONa<br />
Ph<br />
Br<br />
O<br />
64-73%<br />
Br<br />
NaNH 2 /NH 3<br />
Br<br />
PhC≡CH 45-52%<br />
Ph<br />
OTs<br />
aq. OH -<br />
HC≡C<br />
HC≡C-C=CH-CH 3<br />
∆<br />
CH<br />
OH -<br />
3 CH 3<br />
98%<br />
Ionization is favored by<br />
- electron-releasing groups<br />
- good leaving groups<br />
- solvents of high ionizing strength<br />
- stronger bases favor the E1 path over the S N 1 path<br />
The precise nature of the transition state of the E2 mechanism is a function of the<br />
leaving group, and the solvent. E1cb mechanism is not observed with simple alkyl halides<br />
or sulfonates, but with functional groups that capable of stabilizing negative charge on<br />
carbon.<br />
<strong>2.</strong>5 ORIENTATION EFFECTS IN <strong>ELIMINATION</strong> <strong>REACTIONS</strong><br />
The experimental evidence that has been gathered concerning direction of<br />
elimination from substrates reacting by the E1 mechanism indicates that the relative<br />
stability of the product olefins is a major factor in determining direction of elimination.<br />
H<br />
R 2 C δ+ X δ-<br />
+ N(CH 3 ) 3<br />
R 2 HC + X -<br />
A′<br />
B′<br />
Thermodynamically<br />
Controled product<br />
R 2 CHX<br />
A (less-substituted olefin)<br />
B (more-substituted olefin)<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 6
In the E1cb mechanism, the direction of elimination is governed by the kinetic<br />
acidity of the available protons, which, in tern, is determined by the inductive and<br />
resonance effects of nearby substituents and by the degree of steric hindrance to approach<br />
of base to the proton. Preferential proton abstraction from unhindered positions leads to the<br />
formation of less-substituted alkenes, i.e. “Hofmann rule” is followed.<br />
E2 eliminations that proceed through transition states with high double-bond<br />
character give mainly the more substituted olefin because the stability of the olefin is<br />
reflected in the transition state, i.e. said to follow the “Saytzeff rule.”<br />
Base/solvent<br />
+ +<br />
X<br />
X = I MeO - /MeOH 19 63 18<br />
Cl ″ 33 50 17<br />
F ″ 69 21 9<br />
OTs ″ 33 44 23<br />
I t-BuO - /t-BuOH 78 15 7<br />
Cl ″ 91 5 4<br />
F ″ 97 1 1<br />
OTs ″ 83 4 14<br />
Poorer leaving groups favor Hofmann products. Stronger bases favor formation of<br />
the less-substituted olefins. Highly hindered bases favor Hofmann products.<br />
<strong>2.</strong>6 STEREOCHEMISTRY OF E2 <strong>ELIMINATION</strong> <strong>REACTIONS</strong><br />
E2 elimination reaction may proceed via syn or anti mode:<br />
B:<br />
B:<br />
H X<br />
H<br />
syn<br />
anti<br />
C C C=C C C<br />
X<br />
In most cases, E2 elimination proceed via anti mode. Syn elimination is possible,<br />
and becomes dominant mode when special structural features retard anti elimination. In<br />
general trend revealed that anti mode is normally preferred for reactions involving good<br />
leaving groups such as bromide and tosylate, With poorer leaving groups, eg. fluoride,<br />
trimethylamine syn mode becomes important.<br />
D anti<br />
D<br />
H<br />
syn<br />
+ NMe 3<br />
H<br />
H<br />
Ion pair promotes syn elimination of anionic leaving group the syn mode will be<br />
diminished.<br />
H<br />
RO -<br />
C C<br />
H +<br />
X<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 7
The proportion of cis-and trans-isomer of internal olefins formed during<br />
elimination reactions depends on the identity of the leaving group. Halides usually give<br />
predominautly the trans-olefins. Bulkier groups, particularly arenesulfonates, give higher<br />
proportions of the cis-olefin. The normal preference for trans-olefin probably reflects the<br />
greater stability of the trans-olefin; i.e. the unfavorable steric repulsions present in the cistrans<br />
are also present in the E2 transition state leading to cis-olefin. High cis:trans ratio are<br />
attributed to a second steric effect that become important only when the leaving group is<br />
large.<br />
H<br />
R<br />
B:<br />
O<br />
H<br />
O<br />
S-Ar<br />
O<br />
R<br />
H<br />
trans<br />
R<br />
O<br />
S-Ar<br />
O<br />
H<br />
This is favored when the leaving group and base are large<br />
because it permits the bulky base and leaving groups to occupy<br />
positions removed from the alkyl substituents<br />
R<br />
B:<br />
O<br />
H<br />
H<br />
cis<br />
<strong>2.</strong>7 DEHYDRATION OF ALCOHOLS<br />
The dehydration of alcohols take place under acidic conditions involving and E1<br />
mechanism.<br />
slow<br />
H + -H 2 O<br />
-H +<br />
—C—C— —C—C— —C—C— C=C<br />
+<br />
HO H<br />
H 2 O + H H<br />
- Orientation follows the Saytzeff rule<br />
- Relative stability: 3° > 2° > 1° carbocations<br />
- Rearrangement is found<br />
- Exchange of the hydroxyl group with the solvent (S N 1)<br />
- Shows isotope effect at β-deuterated alcohols.<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 8