Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
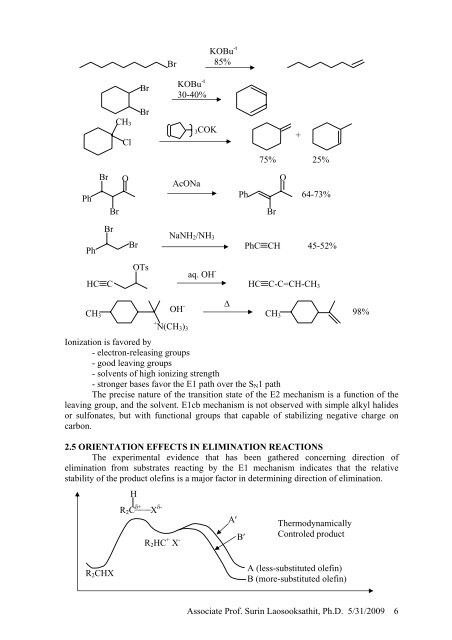
Br<br />
KOBu -t<br />
85%<br />
Br<br />
KOBu -t<br />
30-40%<br />
Br<br />
CH 3<br />
Cl<br />
3COK<br />
+<br />
75% 25%<br />
Ph<br />
Br<br />
Br<br />
O<br />
AcONa<br />
Ph<br />
Br<br />
O<br />
64-73%<br />
Br<br />
NaNH 2 /NH 3<br />
Br<br />
PhC≡CH 45-52%<br />
Ph<br />
OTs<br />
aq. OH -<br />
HC≡C<br />
HC≡C-C=CH-CH 3<br />
∆<br />
CH<br />
OH -<br />
3 CH 3<br />
98%<br />
Ionization is favored by<br />
- electron-releasing groups<br />
- good leaving groups<br />
- solvents of high ionizing strength<br />
- stronger bases favor the E1 path over the S N 1 path<br />
The precise nature of the transition state of the E2 mechanism is a function of the<br />
leaving group, and the solvent. E1cb mechanism is not observed with simple alkyl halides<br />
or sulfonates, but with functional groups that capable of stabilizing negative charge on<br />
carbon.<br />
<strong>2.</strong>5 ORIENTATION EFFECTS IN <strong>ELIMINATION</strong> <strong>REACTIONS</strong><br />
The experimental evidence that has been gathered concerning direction of<br />
elimination from substrates reacting by the E1 mechanism indicates that the relative<br />
stability of the product olefins is a major factor in determining direction of elimination.<br />
H<br />
R 2 C δ+ X δ-<br />
+ N(CH 3 ) 3<br />
R 2 HC + X -<br />
A′<br />
B′<br />
Thermodynamically<br />
Controled product<br />
R 2 CHX<br />
A (less-substituted olefin)<br />
B (more-substituted olefin)<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 6