Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
Chapter 2. POLAR ADDITION AND ELIMINATION REACTIONS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
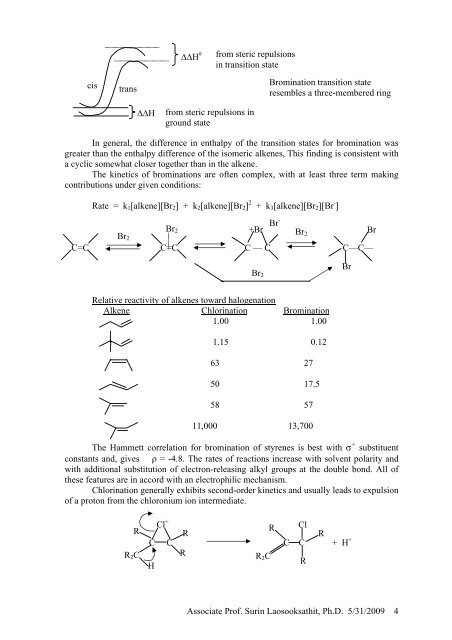
∆∆H #<br />
from steric repulsions<br />
in transition state<br />
cis<br />
trans<br />
Bromination transition state<br />
resembles a three-membered ring<br />
∆∆H<br />
from steric repulsions in<br />
ground state<br />
In general, the difference in enthalpy of the transition states for bromination was<br />
greater than the enthalpy difference of the isomeric alkenes, This finding is consistent with<br />
a cyclic somewhat closer together than in the alkene.<br />
The kinetics of brominations are often complex, with at least three term making<br />
contributions under given conditions:<br />
Rate = k 1 [alkene][Br 2 ] + k 2 [alkene][Br 2 ] 2 + k 3 [alkene][Br 2 ][Br - ]<br />
Br 2<br />
+Br<br />
Br 2<br />
Br 2<br />
Br<br />
Br -<br />
Br<br />
C=C C=C C — C C—C—<br />
Br 2<br />
Relative reactivity of alkenes toward halogenation<br />
Alkene Chlorination Bromination<br />
1.00 1.00<br />
1.15 0.12<br />
63 27<br />
50 17.5<br />
58 57<br />
11,000 13,700<br />
The Hammett correlation for bromination of styrenes is best with σ + substituent<br />
constants and, gives ρ = -4.8. The rates of reactions increase with solvent polarity and<br />
with additional substitution of electron-releasing alkyl groups at the double bond. All of<br />
these features are in accord with an electrophilic mechanism.<br />
Chlorination generally exhibits second-order kinetics and usually leads to expulsion<br />
of a proton from the chloronium ion intermediate.<br />
R<br />
R 2 C<br />
Cl +<br />
R Cl<br />
R<br />
R<br />
C C C C + H +<br />
R<br />
R 2 C<br />
R<br />
H<br />
Associate Prof. Surin Laosooksathit, Ph.D. 5/31/2009 4