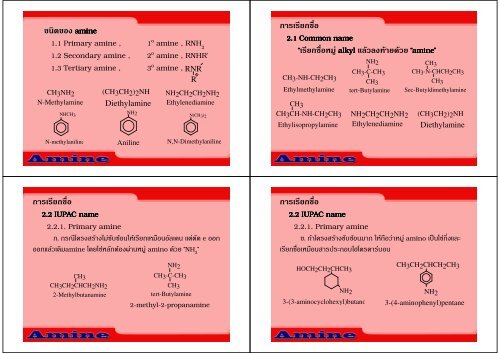
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>amine</strong><br />
<strong>1.1</strong> <strong>Primary</strong> <strong>amine</strong> , 1 o <strong>amine</strong> , <strong>RNH</strong> 2<br />
<strong>1.2</strong> <strong>Secondary</strong> <strong>amine</strong> , 2 o <strong>amine</strong> , <strong>RNH</strong>R’<br />
1.3 Tertiary <strong>amine</strong> , 3 o <strong>amine</strong> , RNR<br />
R<br />
CH3NH2<br />
N-Methyl<strong>amine</strong><br />
NHCH3<br />
(CH3CH2)2NH<br />
Diethyl<strong>amine</strong><br />
NH2<br />
NH2CH2CH2NH2<br />
Ethylenedi<strong>amine</strong><br />
N(CH3)2<br />
กก<br />
2.1 Common name<br />
“ก alkyl “<strong>amine</strong><br />
<strong>amine</strong>”<br />
CH3-NH-CH2CH3<br />
Ethylmethyl<strong>amine</strong><br />
CH3<br />
CH3CH-NH-CH2CH3<br />
Ethylisopropyl<strong>amine</strong><br />
NH2<br />
CH3-C-CH3<br />
CH3<br />
tert-Butyl<strong>amine</strong><br />
NH2CH2CH2NH2<br />
Ethylenedi<strong>amine</strong><br />
CH3<br />
CH3-N-CHCH2CH3<br />
CH3<br />
Sec-Butyldimethyl<strong>amine</strong><br />
(CH3CH2)2NH<br />
Diethyl<strong>amine</strong><br />
N-methylaniline<br />
Aniline<br />
N,N-Dimethylaniline<br />
กก<br />
2.2 IUPAC name<br />
2.2.1. <strong>Primary</strong> <strong>amine</strong><br />
ก. กก e ก<br />
ก<strong>amine</strong> ก amino “NH 2 ”<br />
กก<br />
2.2 IUPAC name<br />
2.2.1. <strong>Primary</strong> <strong>amine</strong><br />
. ก amino ก<br />
กก<br />
CH3<br />
CH3CH2CHCH2NH2<br />
2-Methylbutan<strong>amine</strong><br />
NH2<br />
CH3-C-CH3<br />
CH3<br />
tert-Butyl<strong>amine</strong><br />
2-methyl-2-propan<strong>amine</strong><br />
HOCH2CH2CHCH3<br />
NH2<br />
3-(3-aminocyclohexyl)butanol<br />
CH3CH2CHCH2CH3<br />
NH2<br />
3-(4-aminophenyl)pentane
กก<br />
2.2 IUPAC name<br />
2.2.2 <strong>Secondary</strong> and tertiary <strong>amine</strong><br />
ก. amino ก H<br />
amino N <br />
amino<br />
CH 3 NH- N-methylamino- CH 3 CH 2 NH- N-ethylamino-<br />
กก<br />
2.2 IUPAC name<br />
2.2.2 <strong>Secondary</strong> and tertiary <strong>amine</strong><br />
. กก amino <br />
ก ก<br />
CH3CH2NHCHCH2CH3<br />
CH3CH2NHCH2CH2CH3<br />
1-(N-Ethylamino)propane<br />
CH2CH3<br />
3-(N-Ethylamino)pentane<br />
CH 3 NCH 2 CH 3<br />
N-ethyl-N-methylamion-<br />
CH3NCH3<br />
(CH 3 ) 2 N-<br />
N,N-dimethylamino-<br />
N,N-Dimethylaniline<br />
CH3NHCH2CH3<br />
N-Methylamionethane<br />
กก<br />
2.2 IUPAC name<br />
CH3<br />
CH3CH2NCHCH2CH3<br />
CH2CH3<br />
3-(N-Ethyl-N-methylamino)pentane<br />
N-CH3<br />
กก<br />
2.2 IUPAC name<br />
2.2.3 Quaternary ammonium salt<br />
ก <strong>amine</strong> <br />
Amine ----> Ammonium<br />
Aniline ----> Anilinium<br />
anion<br />
(C 2 H 5 NH 3+ ) 2 SO 4<br />
2-<br />
Ethylammonium sulfate<br />
N-Cyclobutyl-N-methylaniline<br />
(CH 3 ) 3 NH + NO 3<br />
-<br />
C 6 H 5 NH 3+ Cl -<br />
Trimethylammonium nitrate<br />
Anilinium chloride
ÕÕÕ ÕÕÕ<br />
ก<br />
3.1 ¤ÇÒÁÁ<br />
ÁÁÕ<br />
Õ¢ÑéÇ<br />
1 o , 2 o <strong>amine</strong> ÁÕ¢ÑéÇ Â¡àÇé¹ 3 o <strong>amine</strong> ???<br />
3.2 ¡ÒÃ<br />
ÒÃÅÐÅÒÂ<br />
Ò¹éÓ<br />
<strong>amine</strong> กก <br />
<br />
3.3 ก<br />
- hydrocarbon<br />
- alcohol<br />
- carboxylic acid<br />
- <strong>amine</strong> 3 <br />
1 o <strong>amine</strong> > 2 o <strong>amine</strong> > 3 o <strong>amine</strong> à¾ÃÒÐ ?<br />
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
1. Reduction of nitro compounds<br />
Ar-NO2<br />
or<br />
R-NO2<br />
CH3CH2CH2NO2<br />
COOC2H5<br />
H2/Pt<br />
NO2<br />
Fe/H+, Sn/H+ or<br />
H2/Pt or Pd or Ni<br />
Fe/HCl<br />
COOC2H5<br />
NH2<br />
Ar-NH2<br />
or<br />
R-NH2<br />
CH3CH2CH2NH2<br />
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
2. . Reaction of halides with ammonia or <strong>amine</strong>s<br />
R R<br />
R-X R-X R-X R-X<br />
NH3 R-NH2 R-NH R-N<br />
R<br />
R +<br />
R-N-R<br />
R<br />
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
2. . Reaction of halides with ammonia or <strong>amine</strong>s<br />
2.<br />
Cl<br />
NO 2 CH 3 NH 2<br />
?<br />
NO 2<br />
C2H5Cl<br />
NH3<br />
C2H5NH2 CH 3Cl<br />
H<br />
C2H5-N-CH3
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
3. . Reductive amination<br />
Ex<br />
H<br />
CH3C=O + NH2<br />
C=O + NH3<br />
+ <strong>RNH</strong>2<br />
+ R2NH<br />
O<br />
H2/Ni<br />
CH3CCH3 + NH3<br />
H2/Ni or<br />
NaBH3CN CH-NH 2<br />
CH-NHR<br />
CHNR2<br />
NaBH3CN<br />
NH2<br />
CH3-CH-CH3<br />
H<br />
NCH2CH(CH3)2<br />
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
4. . Reduction of nitrile<br />
H2/Ni<br />
R-C N R-CH2NH2<br />
Ex<br />
NaCN<br />
H2/Ni<br />
ClCH2CH2CH2Cl CN(CH2)3CN H2NCH2(CH2)4CH2NH2<br />
CH2Cl CH2CN CH2CH2NH2<br />
NaCN<br />
H2/Ni<br />
¡ÒÃ<br />
ÒÃàµ<br />
àµÃÕÂ<br />
ÃÕÂÁ Amines<br />
5. . Hofmann degradation of amide<br />
O O<br />
R-CNH2 or Ar-CNH2 KOBr R-NH2 or Ar-NH2 + CO3 2-<br />
Ex<br />
O<br />
CH3CH2CH2CNH2<br />
O<br />
CNH2<br />
Br<br />
KOBr<br />
KOBr<br />
NH2<br />
CH3CH2CH2NH2<br />
Br<br />
Home work II<br />
Amines ก<br />
<br />
1<br />
CH3<br />
CH2NH2<br />
?<br />
?<br />
2 CH2=CH2 NH2CH2CH2CH2CH2NH2
ก Amines<br />
1. . salt formation<br />
(CH3)2NH + HNO3 (CH3)2NH2 + NO3 -<br />
NH2 + HCl<br />
NH3 + Cl-<br />
aromatic <strong>amine</strong> ก<br />
ก<br />
NH2<br />
G<br />
G = amino, alkoxide, , methyl <br />
G = nitro, cyano, sulfonic <br />
ก Amines<br />
2. . conversion into amide<br />
Ex:<br />
<strong>RNH</strong>2<br />
O<br />
RCCl<br />
O<br />
O<br />
RC-NHR<br />
O<br />
R2NH RCCl RCNR2<br />
O<br />
R3N<br />
RCCl No reaction<br />
NH2<br />
O<br />
CH3COH<br />
O<br />
NHCCH3<br />
+ H2O<br />
ก Amines<br />
3. . reaction with nitrous acid<br />
HNO 2<br />
ก Amines<br />
3. . reaction with nitrous acid<br />
1. <strong>Primary</strong> aromatic Diazonium salt<br />
2. <strong>Primary</strong> aliphatic <br />
<br />
3. Second aromatic and aliphatic<br />
ArNHR<br />
or<br />
R2NH<br />
NaNO2<br />
HCl<br />
R<br />
Ar-N-N=O<br />
or<br />
R2N-N=O<br />
N-Nitroso<strong>amine</strong><br />
4. Tertiary aromatic<br />
NR2<br />
HONO<br />
O=N<br />
NR2<br />
p-Nitroso compound
ก Amines<br />
3. . reaction with nitrous acid<br />
กก primary aromatic <strong>amine</strong><br />
4. reaction of diazonium salt<br />
NH2 N N+<br />
CuCl<br />
CuBr<br />
CuCN<br />
Cl<br />
Br<br />
CN<br />
KI<br />
I<br />
HCl + NaNO 2<br />
Cl<br />
HNO 2 +<br />
-<br />
NH 2 N N<br />
diazonium salt<br />
HBF4<br />
heat<br />
H2O/H+<br />
H3PO2<br />
H2O<br />
Ar-OH<br />
(Coupling)<br />
(OH,NR2,NHR,NH2)<br />
F<br />
OH<br />
H<br />
N N OH