Poly(2,5-dimethoxyaniline) based pH sensors Nophawan Paradee ...
Poly(2,5-dimethoxyaniline) based pH sensors Nophawan Paradee ...
Poly(2,5-dimethoxyaniline) based pH sensors Nophawan Paradee ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
91 NU Science Journal 2009; 6(S1)<br />
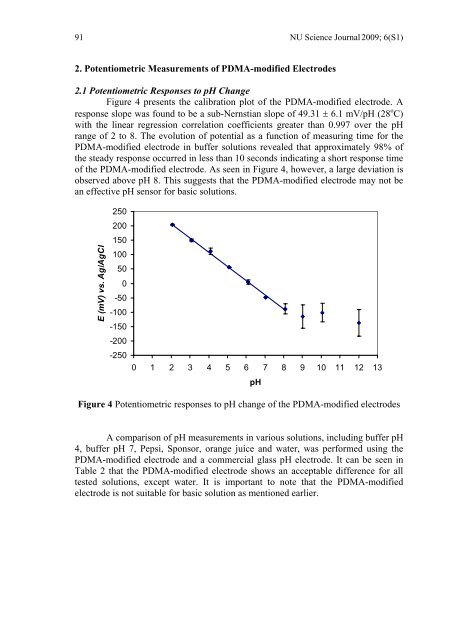
2. Potentiometric Measurements of PDMA-modified Electrodes<br />
2.1 Potentiometric Responses to <strong>pH</strong> Change<br />
Figure 4 presents the calibration plot of the PDMA-modified electrode. A<br />
response slope was found to be a sub-Nernstian slope of 49.31 ± 6.1 mV/<strong>pH</strong> (28 o C)<br />
with the linear regression correlation coefficients greater than 0.997 over the <strong>pH</strong><br />
range of 2 to 8. The evolution of potential as a function of measuring time for the<br />
PDMA-modified electrode in buffer solutions revealed that approximately 98% of<br />
the steady response occurred in less than 10 seconds indicating a short response time<br />
of the PDMA-modified electrode. As seen in Figure 4, however, a large deviation is<br />
observed above <strong>pH</strong> 8. This suggests that the PDMA-modified electrode may not be<br />
an effective <strong>pH</strong> sensor for basic solutions.<br />
E (mV) vs. Ag/AgCl<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0<br />
-50<br />
-100<br />
-150<br />
-200<br />
-250<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13<br />
<strong>pH</strong><br />
Figure 4 Potentiometric responses to <strong>pH</strong> change of the PDMA-modified electrodes<br />
A comparison of <strong>pH</strong> measurements in various solutions, including buffer <strong>pH</strong><br />
4, buffer <strong>pH</strong> 7, Pepsi, Sponsor, orange juice and water, was performed using the<br />
PDMA-modified electrode and a commercial glass <strong>pH</strong> electrode. It can be seen in<br />
Table 2 that the PDMA-modified electrode shows an acceptable difference for all<br />
tested solutions, except water. It is important to note that the PDMA-modified<br />
electrode is not suitable for basic solution as mentioned earlier.