Hydrates and Glycols - NTNU
Hydrates and Glycols - NTNU
Hydrates and Glycols - NTNU
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
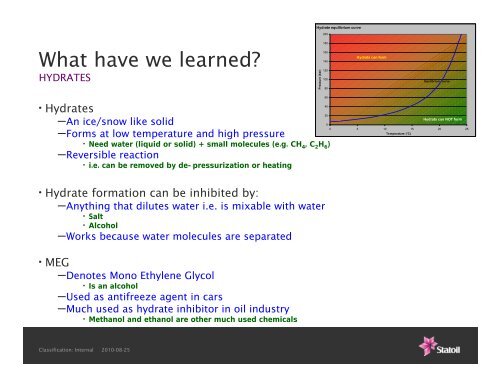
Hydrate equilibrium curve<br />
200<br />
What have we learned?<br />
HYDRATES<br />
Pressure (bar)<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
Hydrats can form<br />
Equilbrium curve<br />
40<br />
•<strong>Hydrates</strong><br />
20<br />
–An ice/snow like solid<br />
0<br />
–Forms at low temperature <strong>and</strong> high pressure<br />
• Need water (liquid or solid) + small molecules (e.g. CH 4 , C 2 H 6 )<br />
–Reversible reaction<br />
• i.e. can be removed by de-pressurization or heating<br />
•Hydrate formation can be inhibited by:<br />
–Anything that dilutes water i.e. is mixable with water<br />
• Salt<br />
• Alcohol<br />
–Works because water molecules are separated<br />
60<br />
Hydrats can NOT form<br />
0 5 10 15 20 25<br />
Temperature (°C)<br />
•MEG<br />
–Denotes Mono Ethylene Glycol<br />
• Is an alcohol<br />
–Used as antifreeze agent in cars<br />
–Much used as hydrate inhibitor in oil industry<br />
• Methanol <strong>and</strong> ethanol are other much used chemicals<br />
Classification: Internal 2010-08-25