- Page 1 and 2:
Synthesis of Chlorogenic Acids & Ch
- Page 3 and 4:
“Imagination is more important th
- Page 5 and 6:
Abbreviations Ac Ac 2 O AcOH MeCN C
- Page 7 and 8:
CONTENT Chapter 1 vi
- Page 9 and 10:
Content Chapter 1 Preparation of ac
- Page 11 and 12:
Content Chapter 1 (1S, 3R, 4R, 5R)-
- Page 13 and 14:
Background Chapter 2 Phenolic Phyto
- Page 15 and 16:
Background Chapter 2 Types of polyp
- Page 17 and 18:
Background Chapter 2 Stilbenes cont
- Page 19 and 20:
Background Chapter 2 Biosynthesis o
- Page 21 and 22:
Background Chapter 2 Biological act
- Page 23 and 24:
Background Chapter 2 terpenoids, to
- Page 25 and 26:
Background Chapter 2 Table 2: Bioav
- Page 27 and 28:
Background Chapter 2 These well kno
- Page 29 and 30:
Background Chapter 2 Hydroxycinnami
- Page 31 and 32:
Background Chapter 2 small amounts
- Page 33 and 34:
Background Chapter 2 Cinnamate tran
- Page 35 and 36:
Background Chapter 2 used in the is
- Page 37 and 38:
Background Chapter 2 acid is implic
- Page 39 and 40:
Background Chapter 2 Chlorogenic ac
- Page 41 and 42:
Background Chapter 2 HO O HO OH C O
- Page 43 and 44:
Background Chapter 2 acyl migration
- Page 45 and 46:
Background Chapter 2 Reported Synth
- Page 47 and 48:
Background Chapter 2 1-O-Cinnamoylq
- Page 49 and 50:
Background Chapter 2 O O O H + CinO
- Page 51 and 52:
Background Chapter 2 chloride gave
- Page 53 and 54:
Background Chapter 2 Synthesis of 1
- Page 55 and 56:
Background Chapter 2 Esterification
- Page 57 and 58:
Background Chapter 2 Synthesis of 3
- Page 59 and 60:
Background Chapter 2 Table 5: Chlor
- Page 61 and 62:
Background Chapter 2 roasting degre
- Page 63 and 64:
Aims and objectives As described ch
- Page 65 and 66:
Results and discussion Chapter 3 Pr
- Page 67 and 68:
Results and discussion Chapter 3 co
- Page 69 and 70:
Results and discussion Chapter 3 Pr
- Page 71 and 72:
Results and discussion Chapter 3 1.
- Page 73 and 74:
Results and discussion Chapter 3 Sy
- Page 75 and 76:
Results and discussion Chapter 3 Ta
- Page 77 and 78:
Results and discussion Chapter 3 Fi
- Page 79 and 80:
Results and discussion Chapter 3 Sy
- Page 81 and 82:
Results and discussion Chapter 3 Fi
- Page 83 and 84:
Results and discussion Chapter 3 Sy
- Page 85 and 86:
Results and discussion Chapter 3 Th
- Page 87 and 88:
Results and discussion Chapter 3 an
- Page 89 and 90:
Results and discussion Chapter 3 20
- Page 91 and 92:
Results and discussion Chapter 3 Th
- Page 93 and 94:
Results and discussion Chapter 3 Ta
- Page 95 and 96:
Results and discussion Chapter 3 Fi
- Page 97 and 98:
Results and discussion Chapter 3 Ge
- Page 99 and 100:
Results and discussion Chapter 3 Sy
- Page 101 and 102:
Results and discussion Chapter 3 Fi
- Page 103 and 104:
Results and discussion Chapter 3 pp
- Page 105 and 106:
Results and discussion Chapter 3 Sy
- Page 107 and 108:
Results and discussion Chapter 3 1
- Page 109 and 110:
Results and discussion Chapter 3 Fi
- Page 111 and 112:
Results and discussion Chapter 3 20
- Page 113 and 114:
Results and discussion Chapter 3 ch
- Page 115 and 116:
Results and discussion Chapter 3 Fi
- Page 117 and 118:
Results and discussion Chapter 3 0
- Page 119 and 120:
Results and discussion Chapter 3 Fi
- Page 121 and 122:
Results and discussion Chapter 3 Th
- Page 123 and 124:
Results and discussion Chapter 3 Sc
- Page 125 and 126:
Results and discussion Chapter 3 Th
- Page 127 and 128:
Results and discussion Chapter 3 di
- Page 129 and 130:
Results and discussion Chapter 3 gr
- Page 131 and 132:
Results and discussion Chapter 3 20
- Page 133 and 134: Results and discussion Chapter 3 Sy
- Page 135 and 136: Results and discussion Chapter 3 pp
- Page 137 and 138: Results and discussion Chapter 3 Sc
- Page 139 and 140: Results and discussion Chapter 3 sp
- Page 141 and 142: Results and discussion Chapter 3 ap
- Page 143 and 144: Results and discussion Chapter 3 Fi
- Page 145 and 146: Results and discussion Chapter 3 In
- Page 147 and 148: Results and discussion Chapter 3 0
- Page 149 and 150: Results and discussion Chapter 3 mu
- Page 151 and 152: Results and discussion Chapter 3 0
- Page 153 and 154: Results and discussion Chapter 3 Th
- Page 155 and 156: Results and discussion Chapter 3 Fi
- Page 157 and 158: Results and discussion Chapter 3 Qu
- Page 159 and 160: Results and discussion Chapter 3 2
- Page 161 and 162: Results and discussion Chapter 3 Fi
- Page 163 and 164: Results and discussion Chapter 3 In
- Page 165 and 166: Results and discussion Chapter 3 0
- Page 167 and 168: Results and discussion Chapter 3 CH
- Page 169 and 170: Results and discussion Chapter 3 0
- Page 171 and 172: Results and discussion Chapter 3 Fi
- Page 173 and 174: Results and discussion Chapter 3 12
- Page 175 and 176: Results and discussion Chapter 3 Sy
- Page 177 and 178: Results and discussion Chapter 3 Fi
- Page 179 and 180: Results and discussion Chapter 3 Fi
- Page 181 and 182: Results and discussion Chapter 3 Al
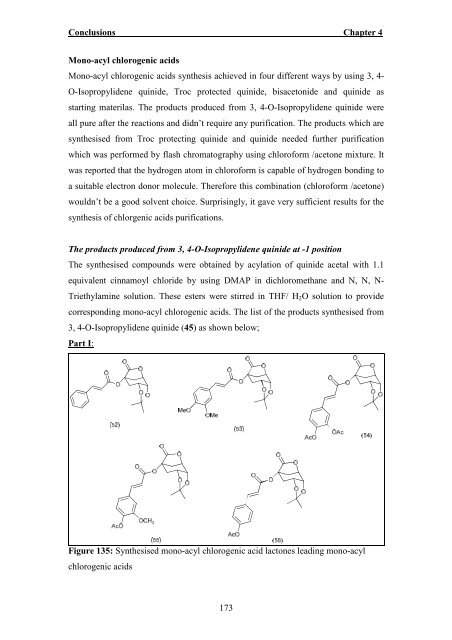
- Page 183: Conclusions Chapter 4 Conclusions A
- Page 187 and 188: Conclusions Chapter 4 The products
- Page 189 and 190: Conclusions Chapter 4 Di-acyl chlor
- Page 191 and 192: Conclusions Chapter 4 Poly-acyl chl
- Page 193 and 194: EXPERIMENTAL Chapter 5 181
- Page 195 and 196: Synthesis of starting materials Qui
- Page 197 and 198: Annex Chapter 6 Acetyl ferulic acid
- Page 199 and 200: Annex Chapter 6 3, 4-dimethoxycinna
- Page 201 and 202: Annex Chapter 6 3, 4-O-Isopropylide
- Page 203 and 204: Annex Chapter 6 1-(β, β, β-trich
- Page 205 and 206: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 207 and 208: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 209 and 210: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 211 and 212: Annex Chapter 6 (1R,3R,4S,5R)-1-dim
- Page 213 and 214: Annex Chapter 6 (1R, 3R, 4S, 5R)-1-
- Page 215 and 216: Annex Chapter 6 (1R, 3R, 4S, 5R)-1-
- Page 217 and 218: Annex Chapter 6 5- diacetylcaffeoyl
- Page 219 and 220: Annex Chapter 6 5- acetyl p-coumaro
- Page 221 and 222: Annex Chapter 6 5-cinnamoyl bisacet
- Page 223 and 224: Annex Chapter 6 5-O-feruloylquinic
- Page 225 and 226: Annex Chapter 6 5-O-dimethoxycinnam
- Page 227 and 228: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 229 and 230: Annex Chapter 6 (1S,3R,4R,5R)-1-(β
- Page 231 and 232: Annex Chapter 6 (1S,3R,4R,5R)-1-(β
- Page 233 and 234: Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 235 and 236:
Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 237 and 238:
Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 239 and 240:
Annex Chapter 6 (1S,3R,4R,5R) 1- (
- Page 241 and 242:
Annex Chapter 6 (1S,3R,4R,5R)-3, 4-
- Page 243 and 244:
Annex Chapter 6 (1S,3R,4R,5R)-3,4-b
- Page 245 and 246:
Annex Chapter 6 (1S,3R,4R,5R)-1,3-d
- Page 247 and 248:
Annex Chapter 6 (1S,3R,4R,5R)-1,3-b
- Page 249 and 250:
Annex Chapter 6 (1S,3R,4R,5R)-1,4-d
- Page 251 and 252:
Annex Chapter 6 Quinide tri-acetate
- Page 253 and 254:
Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 255 and 256:
Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 257 and 258:
Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 259 and 260:
Annex Chapter 6 745 [M+H], 544 [M-C
- Page 261 and 262:
Annex Chapter 6 (C-24), 121.95 (C-3
- Page 263 and 264:
Annex Chapter 6 (acetylferuloyl) qu
- Page 265 and 266:
REFERENCES Chapter 6 253
- Page 267 and 268:
References Chapter 6 21 D. Strack,
- Page 269 and 270:
References Chapter 6 62 K. Herrmann
- Page 271 and 272:
References Chapter 6 104 N. Shibuya
- Page 273 and 274:
References Chapter 6 148 B. Möller
- Page 275 and 276:
References Chapter 6 187 M. Nardini
- Page 277 and 278:
References Chapter 6 221 M. F. Andr
- Page 279 and 280:
References Chapter 6 264 L. Panizzi
- Page 281:
References Chapter 6 303 J. Hucke,