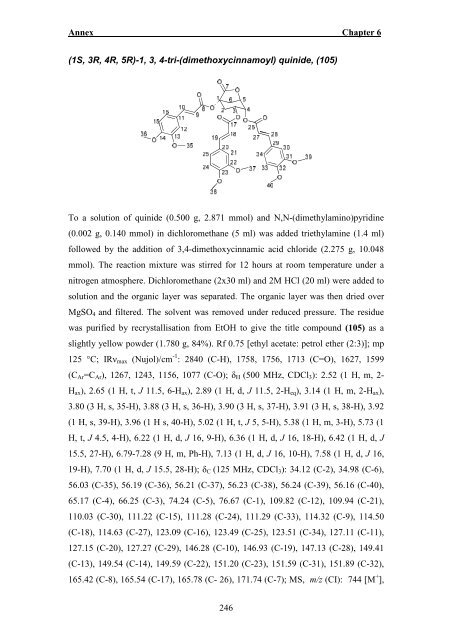
Annex Chapter 6 (1S, 3R, 4R, 5R)-1, 3, 4-tri-(dimethoxycinnamoyl) quinide, (105) To a solution of quinide (0.500 g, 2.871 mmol) and N,N-(dimethylamino)pyridine (0.002 g, 0.140 mmol) in dichloromethane (5 ml) was added triethylamine (1.4 ml) followed by the addition of 3,4-dimethoxycinnamic acid chloride (2.275 g, 10.048 mmol). The reaction mixture was stirred for 12 hours at room temperature under a nitrogen atmosphere. Dichloromethane (2x30 ml) and 2M HCl (20 ml) were added to solution and the organic layer was separated. The organic layer was then dried over MgSO 4 and filtered. The solvent was removed under reduced pressure. The residue was purified by recrystallisation from EtOH to give the title compound (105) as a slightly yellow powder (1.780 g, 84%). Rf 0.75 [ethyl acetate: petrol ether (2:3)]; mp 125 °C; IRν max (Nujol)/cm -1 : 2840 (C-H), 1758, 1756, 1713 (C=O), 1627, 1599 (C Ar =C Ar ), 1267, 1243, 1156, 1077 (C-O); δ H (500 MHz, CDCl 3 ): 2.52 (1 H, m, 2- H ax ), 2.65 (1 H, t, J 11.5, 6-H ax ), 2.89 (1 H, d, J 11.5, 2-H eq ), 3.14 (1 H, m, 2-H ax ), 3.80 (3 H, s, 35-H), 3.88 (3 H, s, 36-H), 3.90 (3 H, s, 37-H), 3.91 (3 H, s, 38-H), 3.92 (1 H, s, 39-H), 3.96 (1 H s, 40-H), 5.02 (1 H, t, J 5, 5-H), 5.38 (1 H, m, 3-H), 5.73 (1 H, t, J 4.5, 4-H), 6.22 (1 H, d, J 16, 9-H), 6.36 (1 H, d, J 16, 18-H), 6.42 (1 H, d, J 15.5, 27-H), 6.79-7.28 (9 H, m, Ph-H), 7.13 (1 H, d, J 16, 10-H), 7.58 (1 H, d, J 16, 19-H), 7.70 (1 H, d, J 15.5, 28-H); δ C (125 MHz, CDCl 3 ): 34.12 (C-2), 34.98 (C-6), 56.<strong>03</strong> (C-35), 56.19 (C-36), 56.21 (C-37), 56.23 (C-38), 56.24 (C-39), 56.16 (C-40), 65.17 (C-4), 66.25 (C-3), 74.24 (C-5), 76.67 (C-1), 109.82 (C-12), 109.94 (C-21), 110.<strong>03</strong> (C-30), 111.22 (C-15), 111.28 (C-24), 111.29 (C-33), 114.32 (C-9), 114.50 (C-18), 114.63 (C-27), 123.09 (C-16), 123.49 (C-25), 123.51 (C-34), 127.11 (C-11), 127.15 (C-20), 127.27 (C-29), 146.28 (C-10), 146.93 (C-19), 147.13 (C-28), 149.41 (C-13), 149.54 (C-14), 149.59 (C-22), 151.20 (C-23), 151.59 (C-31), 151.89 (C-32), 165.42 (C-8), 165.54 (C-17), 165.78 (C- 26), 171.74 (C-7); MS, m/z (CI): 744 [M + ], 246
Annex Chapter 6 745 [M+H], 544 [M-C 11 H 11 O 3 ]; CHN: ( C 40 H 40 O 14 744 g/mol requires: C, 64.52%; H, 5.38%, Found: C, 63.83%; H, 5.48%. (1S, 3R, 4R, 5R)-1, 3, 4-tri-(acetyl p-coumaroyl) quinide, (106) 15 O 35 O 38 16 10 11 12 14 13 O 7 O O 1 6 5 8 O 2 3 4 9 O O O O 17 26 19 18 27 28 29 20 30 25 21 34 31 24 22 33 32 23 O O 36 39 O 37 40 O To a solution of quinide (0.100 g, 0.574 mmol) and N,N-(dimethylamino)pyridine (0.0<strong>03</strong> g, 0.0287 mmol) in dichloromethane (6 ml) was added triethylamine (0.6 ml) followed by the addition of acetyl p-coumaric acid chloride (0.451 g, 2.010 mmol). The reaction mixture was stirred for 16 hours at room temperature and worked up by diluting with dichloromethane (50 ml), washing with 2M HCl (10 ml), NaHCO 3 solution (10 ml) and brine (10 ml). The organic layer was dried over MgSO4 and the solvent was removed under reduced pressure and residue was purified by recrystallisation from EtOH to give the title compound (1S, 3R, 4R, 5R)-1, 3, 4-tri- (acetyl p-coumaroyl) quinide (106) as a white powder (0.395 g, 93%). Rf 0.91 [chloroform: acetone (8:2)]; ν max (KBr)/cm -1 : 1806, 1765, 1721 (C=O), 1635 (C Ar =C Ar ), 1267, 1243, 1156, 1077 (C-O); δ H (400 MHz, CDCl 3 ): 2.29 (3 H, s, 38-H), 2.30 (3 H, s, 39-H), 2.31 (3 H, s, 40-H), 2.55 (1 H, m, 2-H ax ), 2.58 (1 H, dd, J 11.2 and 2.9, 6-H eq ), 2.78 (1 H, d, J 11.6, 2-H eq ), 3.13 (1 H, m, 6-H ax ), 5.02 (1 H, t, J 4.8, 5-H), 5.38 (1 H, m, 3-H), 5.72 (1 H, t, J 4.4, 4-H), 6.26 (1 H, d, J 16, 9-H), 6.38 (1 H, d, J 16, 18-H), 6.44 (1 H, d, J 16, 27-H), 7.05-7.43 (12 H, m, Ph-H), 7.54 (1 H, d, J 15.3, 10-H), 7.56 (1 H, d, J 15.9, 19-H), 7.83 (1 H, d, J 15.3, 28-H); δ C (125 MHz, CDCl 3 ): 21.12 (C-38), 21.24 (C-39), 21.28 (C-40), 34.62 (C-2), 35.86 (C-6), 66.21 (C-4), 66.86 (C-3), 72.66 (C-5), 78.92 (C-1), 112.43 (C-13, C-15), 112.85 (C-22, C- 24), 113.37 (C-31, C-33), 114.81 (C-9), 115.17 (C-18), 115.64 (C-27), 122.31 (C-12, C-16), 122.39 (C-21, C-25), 122.53 (C-30, C-34), 131.82 (C-11), 132.35 (C-20), 247
- Page 1 and 2:
Synthesis of Chlorogenic Acids & Ch
- Page 3 and 4:
“Imagination is more important th
- Page 5 and 6:
Abbreviations Ac Ac 2 O AcOH MeCN C
- Page 7 and 8:
CONTENT Chapter 1 vi
- Page 9 and 10:
Content Chapter 1 Preparation of ac
- Page 11 and 12:
Content Chapter 1 (1S, 3R, 4R, 5R)-
- Page 13 and 14:
Background Chapter 2 Phenolic Phyto
- Page 15 and 16:
Background Chapter 2 Types of polyp
- Page 17 and 18:
Background Chapter 2 Stilbenes cont
- Page 19 and 20:
Background Chapter 2 Biosynthesis o
- Page 21 and 22:
Background Chapter 2 Biological act
- Page 23 and 24:
Background Chapter 2 terpenoids, to
- Page 25 and 26:
Background Chapter 2 Table 2: Bioav
- Page 27 and 28:
Background Chapter 2 These well kno
- Page 29 and 30:
Background Chapter 2 Hydroxycinnami
- Page 31 and 32:
Background Chapter 2 small amounts
- Page 33 and 34:
Background Chapter 2 Cinnamate tran
- Page 35 and 36:
Background Chapter 2 used in the is
- Page 37 and 38:
Background Chapter 2 acid is implic
- Page 39 and 40:
Background Chapter 2 Chlorogenic ac
- Page 41 and 42:
Background Chapter 2 HO O HO OH C O
- Page 43 and 44:
Background Chapter 2 acyl migration
- Page 45 and 46:
Background Chapter 2 Reported Synth
- Page 47 and 48:
Background Chapter 2 1-O-Cinnamoylq
- Page 49 and 50:
Background Chapter 2 O O O H + CinO
- Page 51 and 52:
Background Chapter 2 chloride gave
- Page 53 and 54:
Background Chapter 2 Synthesis of 1
- Page 55 and 56:
Background Chapter 2 Esterification
- Page 57 and 58:
Background Chapter 2 Synthesis of 3
- Page 59 and 60:
Background Chapter 2 Table 5: Chlor
- Page 61 and 62:
Background Chapter 2 roasting degre
- Page 63 and 64:
Aims and objectives As described ch
- Page 65 and 66:
Results and discussion Chapter 3 Pr
- Page 67 and 68:
Results and discussion Chapter 3 co
- Page 69 and 70:
Results and discussion Chapter 3 Pr
- Page 71 and 72:
Results and discussion Chapter 3 1.
- Page 73 and 74:
Results and discussion Chapter 3 Sy
- Page 75 and 76:
Results and discussion Chapter 3 Ta
- Page 77 and 78:
Results and discussion Chapter 3 Fi
- Page 79 and 80:
Results and discussion Chapter 3 Sy
- Page 81 and 82:
Results and discussion Chapter 3 Fi
- Page 83 and 84:
Results and discussion Chapter 3 Sy
- Page 85 and 86:
Results and discussion Chapter 3 Th
- Page 87 and 88:
Results and discussion Chapter 3 an
- Page 89 and 90:
Results and discussion Chapter 3 20
- Page 91 and 92:
Results and discussion Chapter 3 Th
- Page 93 and 94:
Results and discussion Chapter 3 Ta
- Page 95 and 96:
Results and discussion Chapter 3 Fi
- Page 97 and 98:
Results and discussion Chapter 3 Ge
- Page 99 and 100:
Results and discussion Chapter 3 Sy
- Page 101 and 102:
Results and discussion Chapter 3 Fi
- Page 103 and 104:
Results and discussion Chapter 3 pp
- Page 105 and 106:
Results and discussion Chapter 3 Sy
- Page 107 and 108:
Results and discussion Chapter 3 1
- Page 109 and 110:
Results and discussion Chapter 3 Fi
- Page 111 and 112:
Results and discussion Chapter 3 20
- Page 113 and 114:
Results and discussion Chapter 3 ch
- Page 115 and 116:
Results and discussion Chapter 3 Fi
- Page 117 and 118:
Results and discussion Chapter 3 0
- Page 119 and 120:
Results and discussion Chapter 3 Fi
- Page 121 and 122:
Results and discussion Chapter 3 Th
- Page 123 and 124:
Results and discussion Chapter 3 Sc
- Page 125 and 126:
Results and discussion Chapter 3 Th
- Page 127 and 128:
Results and discussion Chapter 3 di
- Page 129 and 130:
Results and discussion Chapter 3 gr
- Page 131 and 132:
Results and discussion Chapter 3 20
- Page 133 and 134:
Results and discussion Chapter 3 Sy
- Page 135 and 136:
Results and discussion Chapter 3 pp
- Page 137 and 138:
Results and discussion Chapter 3 Sc
- Page 139 and 140:
Results and discussion Chapter 3 sp
- Page 141 and 142:
Results and discussion Chapter 3 ap
- Page 143 and 144:
Results and discussion Chapter 3 Fi
- Page 145 and 146:
Results and discussion Chapter 3 In
- Page 147 and 148:
Results and discussion Chapter 3 0
- Page 149 and 150:
Results and discussion Chapter 3 mu
- Page 151 and 152:
Results and discussion Chapter 3 0
- Page 153 and 154:
Results and discussion Chapter 3 Th
- Page 155 and 156:
Results and discussion Chapter 3 Fi
- Page 157 and 158:
Results and discussion Chapter 3 Qu
- Page 159 and 160:
Results and discussion Chapter 3 2
- Page 161 and 162:
Results and discussion Chapter 3 Fi
- Page 163 and 164:
Results and discussion Chapter 3 In
- Page 165 and 166:
Results and discussion Chapter 3 0
- Page 167 and 168:
Results and discussion Chapter 3 CH
- Page 169 and 170:
Results and discussion Chapter 3 0
- Page 171 and 172:
Results and discussion Chapter 3 Fi
- Page 173 and 174:
Results and discussion Chapter 3 12
- Page 175 and 176:
Results and discussion Chapter 3 Sy
- Page 177 and 178:
Results and discussion Chapter 3 Fi
- Page 179 and 180:
Results and discussion Chapter 3 Fi
- Page 181 and 182:
Results and discussion Chapter 3 Al
- Page 183 and 184:
Conclusions Chapter 4 Conclusions A
- Page 185 and 186:
Conclusions Chapter 4 Mono-acyl chl
- Page 187 and 188:
Conclusions Chapter 4 The products
- Page 189 and 190:
Conclusions Chapter 4 Di-acyl chlor
- Page 191 and 192:
Conclusions Chapter 4 Poly-acyl chl
- Page 193 and 194:
EXPERIMENTAL Chapter 5 181
- Page 195 and 196:
Synthesis of starting materials Qui
- Page 197 and 198:
Annex Chapter 6 Acetyl ferulic acid
- Page 199 and 200:
Annex Chapter 6 3, 4-dimethoxycinna
- Page 201 and 202:
Annex Chapter 6 3, 4-O-Isopropylide
- Page 203 and 204:
Annex Chapter 6 1-(β, β, β-trich
- Page 205 and 206:
Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 207 and 208: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 209 and 210: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 211 and 212: Annex Chapter 6 (1R,3R,4S,5R)-1-dim
- Page 213 and 214: Annex Chapter 6 (1R, 3R, 4S, 5R)-1-
- Page 215 and 216: Annex Chapter 6 (1R, 3R, 4S, 5R)-1-
- Page 217 and 218: Annex Chapter 6 5- diacetylcaffeoyl
- Page 219 and 220: Annex Chapter 6 5- acetyl p-coumaro
- Page 221 and 222: Annex Chapter 6 5-cinnamoyl bisacet
- Page 223 and 224: Annex Chapter 6 5-O-feruloylquinic
- Page 225 and 226: Annex Chapter 6 5-O-dimethoxycinnam
- Page 227 and 228: Annex Chapter 6 (1S, 3R, 4R, 5R)-1-
- Page 229 and 230: Annex Chapter 6 (1S,3R,4R,5R)-1-(β
- Page 231 and 232: Annex Chapter 6 (1S,3R,4R,5R)-1-(β
- Page 233 and 234: Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 235 and 236: Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 237 and 238: Annex Chapter 6 (1S, 3R, 4R, 5R)-3-
- Page 239 and 240: Annex Chapter 6 (1S,3R,4R,5R) 1- (
- Page 241 and 242: Annex Chapter 6 (1S,3R,4R,5R)-3, 4-
- Page 243 and 244: Annex Chapter 6 (1S,3R,4R,5R)-3,4-b
- Page 245 and 246: Annex Chapter 6 (1S,3R,4R,5R)-1,3-d
- Page 247 and 248: Annex Chapter 6 (1S,3R,4R,5R)-1,3-b
- Page 249 and 250: Annex Chapter 6 (1S,3R,4R,5R)-1,4-d
- Page 251 and 252: Annex Chapter 6 Quinide tri-acetate
- Page 253 and 254: Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 255 and 256: Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 257: Annex Chapter 6 (1S, 3R, 4R, 5R)-1,
- Page 261 and 262: Annex Chapter 6 (C-24), 121.95 (C-3
- Page 263 and 264: Annex Chapter 6 (acetylferuloyl) qu
- Page 265 and 266: REFERENCES Chapter 6 253
- Page 267 and 268: References Chapter 6 21 D. Strack,
- Page 269 and 270: References Chapter 6 62 K. Herrmann
- Page 271 and 272: References Chapter 6 104 N. Shibuya
- Page 273 and 274: References Chapter 6 148 B. Möller
- Page 275 and 276: References Chapter 6 187 M. Nardini
- Page 277 and 278: References Chapter 6 221 M. F. Andr
- Page 279 and 280: References Chapter 6 264 L. Panizzi
- Page 281: References Chapter 6 303 J. Hucke,