pdf download
pdf download
pdf download
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
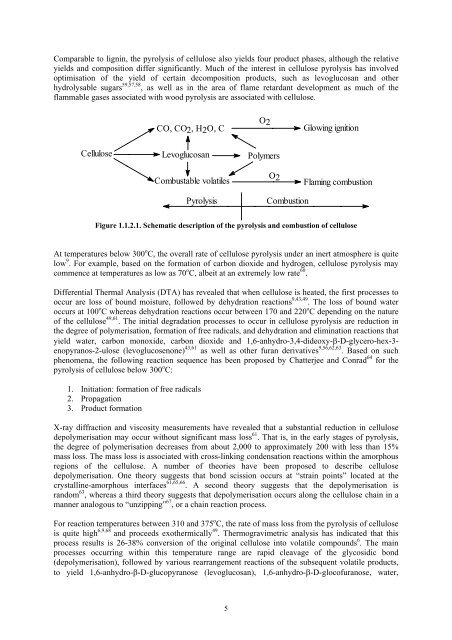
Comparable to lignin, the pyrolysis of cellulose also yields four product phases, although the relative<br />
yields and composition differ significantly. Much of the interest in cellulose pyrolysis has involved<br />
optimisation of the yield of certain decomposition products, such as levoglucosan and other<br />
hydrolysable sugars 59,57,58 , as well as in the area of flame retardant development as much of the<br />
flammable gases associated with wood pyrolysis are associated with cellulose.<br />
CO, CO2, H2O, C<br />
O2<br />
Glowing ignition<br />
Cellulose<br />
Levoglucosan<br />
Polymers<br />
Combustable volatiles<br />
O2<br />
Flaming combustion<br />
Pyrolysis<br />
Combustion<br />
Figure 1.1.2.1. Schematic description of the pyrolysis and combustion of cellulose<br />
At temperatures below 300 o C, the overall rate of cellulose pyrolysis under an inert atmosphere is quite<br />
low 9 . For example, based on the formation of carbon dioxide and hydrogen, cellulose pyrolysis may<br />
commence at temperatures as low as 70 o C, albeit at an extremely low rate 60 .<br />
Differential Thermal Analysis (DTA) has revealed that when cellulose is heated, the first processes to<br />
occur are loss of bound moisture, followed by dehydration reactions 9,43,49 . The loss of bound water<br />
occurs at 100 o C whereas dehydration reactions occur between 170 and 220 o C depending on the nature<br />
of the cellulose 49,61 . The initial degradation processes to occur in cellulose pyrolysis are reduction in<br />
the degree of polymerisation, formation of free radicals, and dehydration and elimination reactions that<br />
yield water, carbon monoxide, carbon dioxide and 1,6-anhydro-3,4-dideoxy-β-D-glycero-hex-3-<br />
enopyranos-2-ulose (levoglucosenone) 43,61 as well as other furan derivatives 9,56,62,63 . Based on such<br />
phenomena, the following reaction sequence has been proposed by Chatterjee and Conrad 64 for the<br />
pyrolysis of cellulose below 300 o C:<br />
1. Initiation: formation of free radicals<br />
2. Propagation<br />
3. Product formation<br />
X-ray diffraction and viscosity measurements have revealed that a substantial reduction in cellulose<br />
depolymerisation may occur without significant mass loss 61 . That is, in the early stages of pyrolysis,<br />
the degree of polymerisation decreases from about 2,000 to approximately 200 with less than 15%<br />
mass loss. The mass loss is associated with cross-linking condensation reactions within the amorphous<br />
regions of the cellulose. A number of theories have been proposed to describe cellulose<br />
depolymerisation. One theory suggests that bond scission occurs at “strain points” located at the<br />
crystalline-amorphous interfaces 61,65,66 . A second theory suggests that the depolymerisation is<br />
random 63 , whereas a third theory suggests that depolymerisation occurs along the cellulose chain in a<br />
manner analogous to “unzipping” 67 , or a chain reaction process.<br />
For reaction temperatures between 310 and 375 o C, the rate of mass loss from the pyrolysis of cellulose<br />
is quite high 6,9,68 and proceeds exothermically 49 . Thermogravimetric analysis has indicated that this<br />
process results is 26-38% conversion of the original cellulose into volatile compounds 6 . The main<br />
processes occurring within this temperature range are rapid cleavage of the glycosidic bond<br />
(depolymerisation), followed by various rearrangement reactions of the subsequent volatile products,<br />
to yield 1,6-anhydro-β-D-glucopyranose (levoglucosan), 1,6-anhydro-β-D-glocofuranose, water,<br />
5