FLUCILONE Cream - Lomus Pharmaceuticals Pvt. Ltd.
FLUCILONE Cream - Lomus Pharmaceuticals Pvt. Ltd.
FLUCILONE Cream - Lomus Pharmaceuticals Pvt. Ltd.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
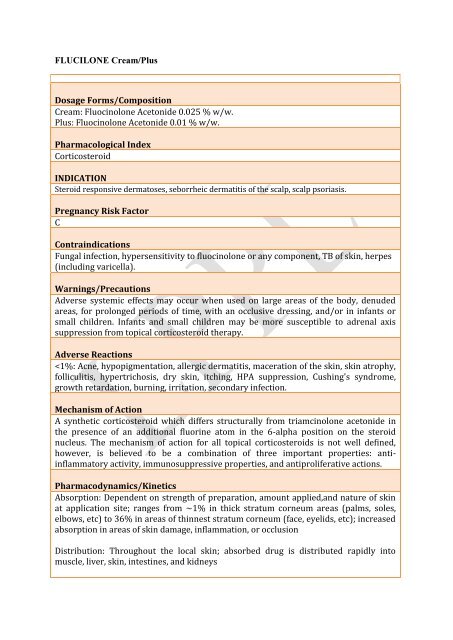
<strong>FLUCILONE</strong> <strong>Cream</strong>/Plus<br />
Dosage Forms/Composition<br />
<strong>Cream</strong>: Fluocinolone Acetonide 0.025 % w/w.<br />
Plus: Fluocinolone Acetonide 0.01 % w/w.<br />
Pharmacological Index<br />
Corticosteroid<br />
INDICATION<br />
Steroid responsive dermatoses, seborrheic dermatitis of the scalp, scalp psoriasis.<br />
Pregnancy Risk Factor<br />
C<br />
Contraindications<br />
Fungal infection, hypersensitivity to fluocinolone or any component, TB of skin, herpes<br />
(including varicella).<br />
Warnings/Precautions<br />
Adverse systemic effects may occur when used on large areas of the body, denuded<br />
areas, for prolonged periods of time, with an occlusive dressing, and/or in infants or<br />
small children. Infants and small children may be more susceptible to adrenal axis<br />
suppression from topical corticosteroid therapy.<br />
Adverse Reactions<br />
Metabolism: Primarily in the skin; small amount absorbed into systemic circulation is<br />
metabolized primarily in the liver to inactive compounds<br />
Elimination: By the kidneys primarily as glucuronides and sulfate, but also as<br />
unconjugated products; small amounts of metabolites are excreted in feces<br />
Usual Dosage<br />
Children and Adults: Topical: Apply a thin layer to affected area 2-4 times/day. Therapy<br />
should be discontinued when control is achieved; if no improvement is seen,<br />
reassessment of diagnosis may be necessary.<br />
Patient Information<br />
A thin film of cream or ointment is effective; do not overuse; do not use tight-fitting<br />
diapers or plastic pants on children being treated in the diaper area; use only as<br />
prescribed, and for no longer than the period prescribed; apply sparingly in light film;<br />
rub in lightly; avoid contact with eyes; notify physician if condition being treated<br />
persists or worsens.<br />
LOMUS Drug Information Center<br />
<strong>Lomus</strong> <strong>Pharmaceuticals</strong> <strong>Pvt</strong>. <strong>Ltd</strong>.<br />
P.O. Box No 4506, <strong>Lomus</strong> House (Corporate office),<br />
Kailash Chour, Lazimpat, Kathmandu, Nepal<br />
Ph: 4436396 (Hunting Line). Fx: 977-1-4436395<br />
E-mail: druginfo@lomus.com.np