OUCOM DEPARTMENT OF FAMILY MEDICINE 2010-2011 AN
OUCOM DEPARTMENT OF FAMILY MEDICINE 2010-2011 AN
OUCOM DEPARTMENT OF FAMILY MEDICINE 2010-2011 AN
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>OUCOM</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>FAMILY</strong> <strong>MEDICINE</strong><br />
<strong>2010</strong>-<strong>2011</strong> <strong>AN</strong>NUAL REPORT<br />
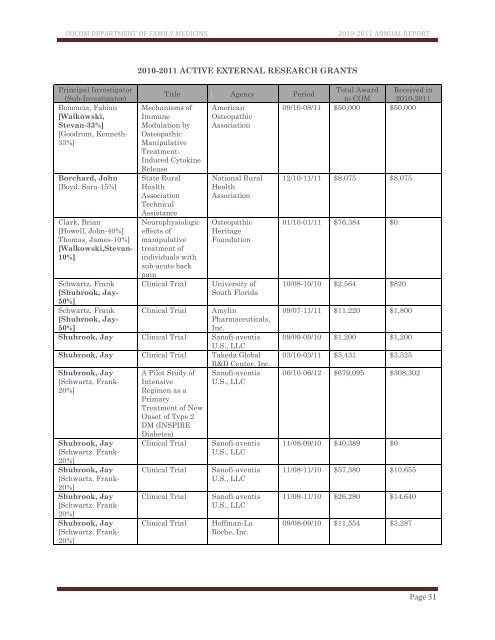
Principal Investigator<br />
(Sub-Investigator)<br />
Benencia, Fabian<br />
[Walkowski,<br />
Stevan-33%]<br />
[Goodrum, Kenneth-<br />
33%]<br />
Borchard, John<br />
[Boyd, Sara-15%]<br />
Clark, Brian<br />
[Howell, John-40%]<br />
Thomas, James-10%]<br />
[Walkowski,Stevan-<br />
10%]<br />
Schwartz, Frank<br />
[Shubrook, Jay-<br />
50%]<br />
Schwartz, Frank<br />
[Shubrook, Jay-<br />
50%]<br />
<strong>2010</strong>-<strong>2011</strong> ACTIVE EXTERNAL RESEARCH GR<strong>AN</strong>TS<br />
Title Agency Period<br />
Total Award Received in<br />
to COM <strong>2010</strong>-<strong>2011</strong><br />
Mechanisms of American<br />
09/10-08/11 $50,000 $50,000<br />
Immune<br />
Osteopathic<br />
Modulation by Association<br />
Osteopathic<br />
Manipulative<br />
Treatment-<br />
Induced Cytokine<br />
Release<br />
State Rural<br />
Health<br />
Association<br />
Technical<br />
Assistance<br />
Neurophysiologic<br />
effects of<br />
manipulative<br />
treatment of<br />
individuals with<br />
sub-acute back<br />
pain<br />
Clinical Trial<br />
Clinical Trial<br />
National Rural<br />
Health<br />
Association<br />
Osteopathic<br />
Heritage<br />
Foundation<br />
University of<br />
South Florida<br />
Amylin<br />
Pharmaceuticals,<br />
Inc.<br />
Shubrook, Jay Clinical Trial Sanofi-aventis<br />
U.S., LLC<br />
Shubrook, Jay Clinical Trial Takeda Global<br />
R&D Center, Inc.<br />
Shubrook, Jay A Pilot Study of Sanofi-aventis<br />
[Schwartz, Frank- Intensive<br />
U.S., LLC<br />
20%]<br />
Regimen as a<br />
Primary<br />
Treatment of New<br />
Onset of Type 2<br />
DM (INSPIRE<br />
Diabetes)<br />
Shubrook, Jay<br />
[Schwartz, Frank-<br />
20%]<br />
Shubrook, Jay<br />
[Schwartz, Frank-<br />
20%]<br />
Shubrook, Jay<br />
[Schwartz, Frank-<br />
20%]<br />
Shubrook, Jay<br />
[Schwartz, Frank-<br />
20%]<br />
Clinical Trial<br />
Clinical Trial<br />
Clinical Trial<br />
Clinical Trial<br />
Sanofi-aventis<br />
U.S., LLC<br />
Sanofi-aventis<br />
U.S., LLC<br />
Sanofi-aventis<br />
U.S., LLC<br />
Hoffman-La<br />
Roche, Inc.<br />
12/10-11/11 $8,075 $8,075<br />
01/10-01/11 $76,384 $0<br />
10/08-10/10 $2,564 $820<br />
09/07-11/11 $11,220 $1,800<br />
09/09-09/10 $1,200 $1,200<br />
03/10-03/11 $5,431 $3,525<br />
06/10-06/12 $679,095 $308,302<br />
11/08-09/10 $40,389 $0<br />
11/08-11/10 $57,380 $10,655<br />
11/09-11/10 $26,280 $14,640<br />
09/08-09/10 $11,554 $3,287<br />
Page 31