After Heparin: - The Pew Charitable Trusts
After Heparin: - The Pew Charitable Trusts
After Heparin: - The Pew Charitable Trusts
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
50<br />
0<br />
INTRODUCTION AND BACKGROUND<br />
unassessable. 42,43 Once the products were removed from the market, reports of unusual adverse reactions<br />
essentially ceased. 44 It was later discovered that the adulteration of heparin had occurred during manufacture<br />
in China. 45<br />
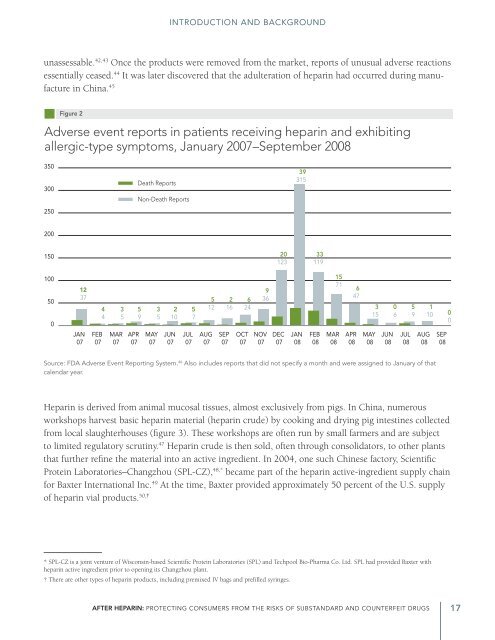
Figure 2<br />
Adverse event reports in patients receiving heparin and exhibiting<br />
allergic-type symptoms, January 2007–September 2008<br />
350<br />
300<br />
250<br />
Death Reports<br />
Non-Death Reports<br />
39<br />
315<br />
200<br />
150<br />
20<br />
123<br />
33<br />
119<br />
100<br />
50<br />
0<br />
12<br />
37<br />
JAN<br />
07<br />
FEB<br />
07<br />
4<br />
4<br />
3<br />
5<br />
MAR<br />
07<br />
APR<br />
07<br />
5<br />
9<br />
MAY<br />
07<br />
3<br />
5<br />
2<br />
10<br />
JUN<br />
07<br />
JUL<br />
07<br />
5<br />
7<br />
5<br />
12<br />
AUG<br />
07<br />
2<br />
16<br />
SEP<br />
07<br />
6<br />
24<br />
OCT<br />
07<br />
9<br />
36<br />
NOV<br />
07<br />
DEC<br />
07<br />
JAN<br />
08<br />
FEB<br />
08<br />
15<br />
71<br />
MAR<br />
08<br />
6<br />
47<br />
APR<br />
08<br />
3<br />
15<br />
MAY<br />
08<br />
JUN<br />
08<br />
0<br />
6<br />
JUL<br />
08<br />
5<br />
9<br />
AUG<br />
08<br />
1<br />
10 0<br />
0<br />
SEP<br />
08<br />
Source: FDA Adverse Event Reporting System. 46 Also includes reports that did not specify a month and were assigned to January of that<br />
calendar year.<br />
<strong>Heparin</strong> is derived from animal mucosal tissues, almost exclusively from pigs. In China, numerous<br />
workshops harvest basic heparin material (heparin crude) by cooking and drying pig intestines collected<br />
from local slaughterhouses (figure 3). <strong>The</strong>se workshops are often run by small farmers and are subject<br />
to limited regulatory scrutiny. 47 <strong>Heparin</strong> crude is then sold, often through consolidators, to other plants<br />
that further refine the material into an active ingredient. In 2004, one such Chinese factory, Scientific<br />
Protein Laboratories–Changzhou (SPL-CZ), 48,* became part of the heparin active-ingredient supply chain<br />
for Baxter International Inc. 49 At the time, Baxter provided approximately 50 percent of the U.S. supply<br />
of heparin vial products. 50,†<br />
* SPL-CZ is a joint venture of Wisconsin-based Scientific Protein Laboratories (SPL) and Techpool Bio-Pharma Co. Ltd. SPL had provided Baxter with<br />
heparin active ingredient prior to opening its Changzhou plant.<br />
† <strong>The</strong>re are other types of heparin products, including premixed IV bags and prefilled syringes.<br />
<strong>After</strong> <strong>Heparin</strong>: PRotecting Consumers from the Risks of Substandard and Counterfeit Drugs 17