Communicating the Value of Pharmacodynamic Modelling in Drug ...
Communicating the Value of Pharmacodynamic Modelling in Drug ...
Communicating the Value of Pharmacodynamic Modelling in Drug ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
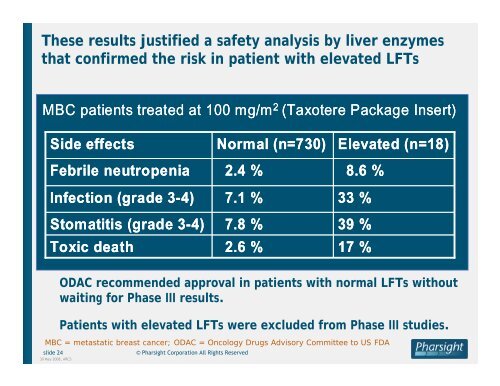
These results justified a safety analysis by liver enzymes<br />
that confirmed <strong>the</strong> risk <strong>in</strong> patient with elevated LFTs<br />
MBC patients treated at 100 mg/m 2 (Taxotere Package Insert)<br />
Side effects<br />
Febrile neutropenia<br />
Infection (grade 3-4)<br />
Stomatitis (grade 3-4)<br />
Toxic death<br />
Normal (n=730)<br />
2.4 %<br />
7.1 %<br />
7.8 %<br />
2.6 %<br />
Elevated (n=18)<br />
8.6 %<br />
33 %<br />
39 %<br />
17 %<br />
ODAC recommended approval <strong>in</strong> patients with normal LFTs without<br />
wait<strong>in</strong>g for Phase III results.<br />
Patients with elevated LFTs were excluded from Phase III studies.<br />
MBC = metastatic breast cancer; ODAC = Oncology <strong>Drug</strong>s Advisory Committee to US FDA<br />
slide 24<br />
30 May 2008, ARCS<br />
© Pharsight Corporation All Rights Reserved