Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
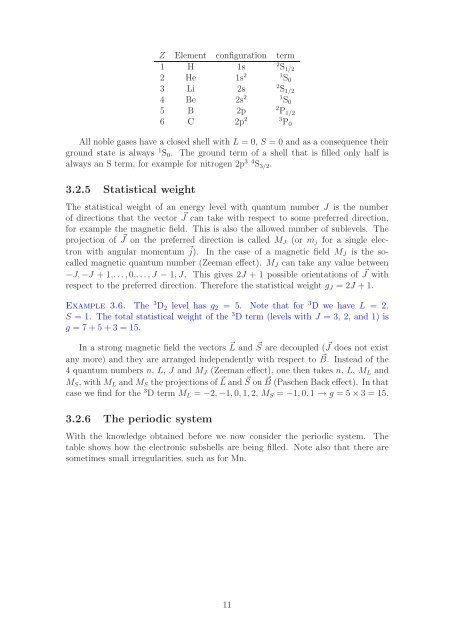
Z Element configuration term<br />
1 H 1s 2 S 1/2<br />
2 He 1s 2 1 S 0<br />
3 Li 2s 2 S 1/2<br />
4 Be 2s 2 1 S 0<br />
5 B 2p 2 P 1/2<br />
6 C 2p 2 3 P 0<br />
All noble gases have a closed shell with L = 0, S = 0 and as a consequence their<br />
ground state is always 1 S 0 . The ground term of a shell that is filled only half is<br />
always an S term, for example for nitrogen 2p 3 4 S 3/2 .<br />
3.2.5 Statistical weight<br />
The statistical weight of an energy level with quantum number J is the number<br />
of directions that the vector ⃗ J can take with respect to some preferred direction,<br />
for example the magnetic field. This is also the allowed number of sublevels. The<br />
projection of ⃗ J on the preferred direction is called MJ (or m j for a single electron<br />
with angular momentum ⃗j). In the case of a magnetic field M J is the socalled<br />
magnetic quantum number (Zeeman effect). M J can take any value between<br />
−J, −J + 1,...,0,...,J − 1, J. This gives 2J + 1 possible orientations of ⃗ J with<br />
respect to the preferred direction. Therefore the statistical weight g J = 2J + 1.<br />
Example 3.6. The 3 D 2 level has g 2 = 5. Note that for 3 D we have L = 2,<br />
S = 1. The total statistical weight of the 3 D term (levels with J = 3, 2, and 1) is<br />
g = 7 + 5 + 3 = 15.<br />
In a strong magnetic field the vectors ⃗ L and ⃗ S are decoupled ( ⃗ J does not exist<br />
any more) and they are arranged independently with respect to ⃗ B. Instead of the<br />
4 quantum numbers n, L, J and M J (Zeeman effect), one then takes n, L, M L and<br />
M S , with M L and M S the projections of ⃗ L and ⃗ S on ⃗ B (Paschen Back effect). In that<br />
case we find for the 3 D term M L = −2, −1, 0, 1, 2, M S = −1, 0, 1 → g = 5 × 3 = 15.<br />
3.2.6 The periodic system<br />
With the knowledge obtained before we now consider the periodic system. The<br />
table shows how the electronic subshells are being filled. Note also that there are<br />
sometimes small irregularities, such as for Mn.<br />
11