Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3.6.7 Photoionisation<br />
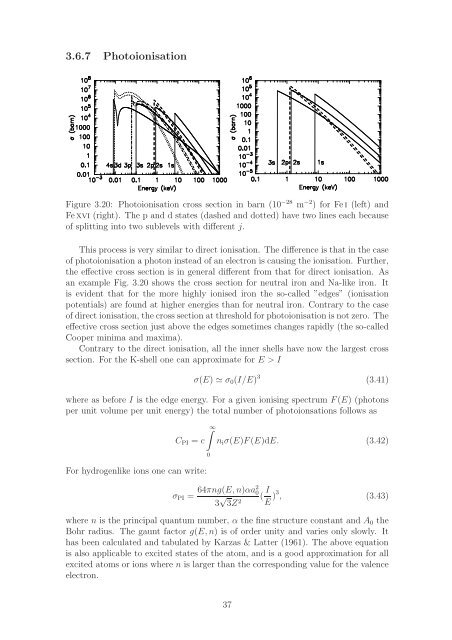
Figure 3.20: Photoionisation cross section in barn (10 −28 m −2 ) for Fei (left) and<br />
Fexvi (right). The p and d states (dashed and dotted) have two lines each because<br />
of splitting into two sublevels with different j.<br />
This process is very similar to direct ionisation. The difference is that in the case<br />
of photoionisation a photon instead of an electron is causing the ionisation. Further,<br />
the effective cross section is in general different from that for direct ionisation. As<br />
an example Fig. 3.20 shows the cross section for neutral iron and Na-like iron. It<br />
is evident that for the more highly ionised iron the so-called ”edges” (ionisation<br />
potentials) are found at higher energies than for neutral iron. Contrary to the case<br />
of direct ionisation, the cross section at threshold for photoionisation is not zero. The<br />
effective cross section just above the edges sometimes changes rapidly (the so-called<br />
Cooper minima and maxima).<br />
Contrary to the direct ionisation, all the inner shells have now the largest cross<br />
section. For the K-shell one can approximate for E > I<br />
σ(E) ≃ σ 0 (I/E) 3 (3.41)<br />
where as before I is the edge energy. For a given ionising spectrum F(E) (photons<br />
per unit volume per unit energy) the total number of photoionsations follows as<br />
C PI = c<br />
For hydrogenlike ions one can write:<br />
∫ ∞<br />
0<br />
n i σ(E)F(E)dE. (3.42)<br />
σ PI = 64πng(E, n)αa2 0<br />
3 √ 3Z 2 ( I E )3 , (3.43)<br />
where n is the principal quantum number, α the fine structure constant and A 0 the<br />
Bohr radius. The gaunt factor g(E, n) is of order unity and varies only slowly. It<br />
has been calculated and tabulated by Karzas & Latter (1961). The above equation<br />
is also applicable to excited states of the atom, and is a good approximation for all<br />
excited atoms or ions where n is larger than the corresponding value for the valence<br />
electron.<br />
37