Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
Thermal X-ray radiation (PDF) - SRON
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3.6.5 Collisional ionisation<br />
Collisional ionisation occurs when during the interaction of a free electron with an<br />
atom or ion the free electron transfers a part of its energy to one of the bound<br />
electrons, which is then able to escape from the ion. A necessary condition is that<br />
the kinetic energy E of the free electron must be larger than the binding energy I<br />
of the atomic shell from which the bound electron escapes.<br />
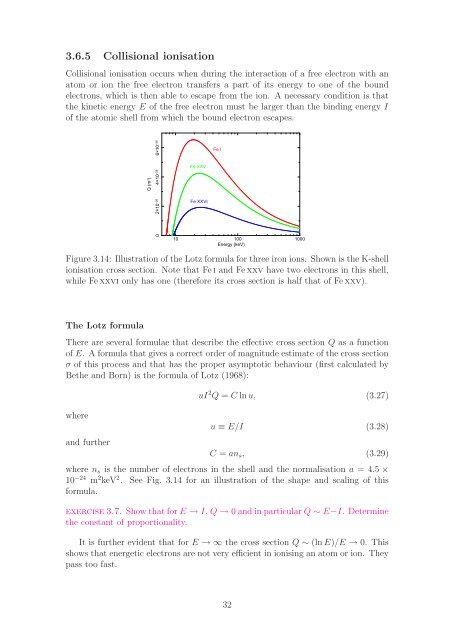
Q (m 2 )<br />
0 2×10 −26 4×10 −26 6×10 −26<br />
Fe I<br />
Fe XXV<br />
Fe XXVI<br />
10 100 1000<br />
Energy (keV)<br />
Figure 3.14: Illustration of the Lotz formula for three iron ions. Shown is the K-shell<br />
ionisation cross section. Note that Fei and Fexxv have two electrons in this shell,<br />
while Fexxvi only has one (therefore its cross section is half that of Fexxv).<br />
The Lotz formula<br />
There are several formulae that describe the effective cross section Q as a function<br />
of E. A formula that gives a correct order of magnitude estimate of the cross section<br />
σ of this process and that has the proper asymptotic behaviour (first calculated by<br />
Bethe and Born) is the formula of Lotz (1968):<br />
where<br />
and further<br />
uI 2 Q = C ln u, (3.27)<br />
u ≡ E/I (3.28)<br />
C = an s , (3.29)<br />
where n s is the number of electrons in the shell and the normalisation a = 4.5 ×<br />
10 −24 m 2 keV 2 . See Fig. 3.14 for an illustration of the shape and scaling of this<br />
formula.<br />
exercise 3.7. Show that for E → I, Q → 0 and in particular Q ∼ E−I. Determine<br />
the constant of proportionality.<br />
It is further evident that for E → ∞ the cross section Q ∼ (ln E)/E → 0. This<br />
shows that energetic electrons are not very efficient in ionising an atom or ion. They<br />
pass too fast.<br />
32